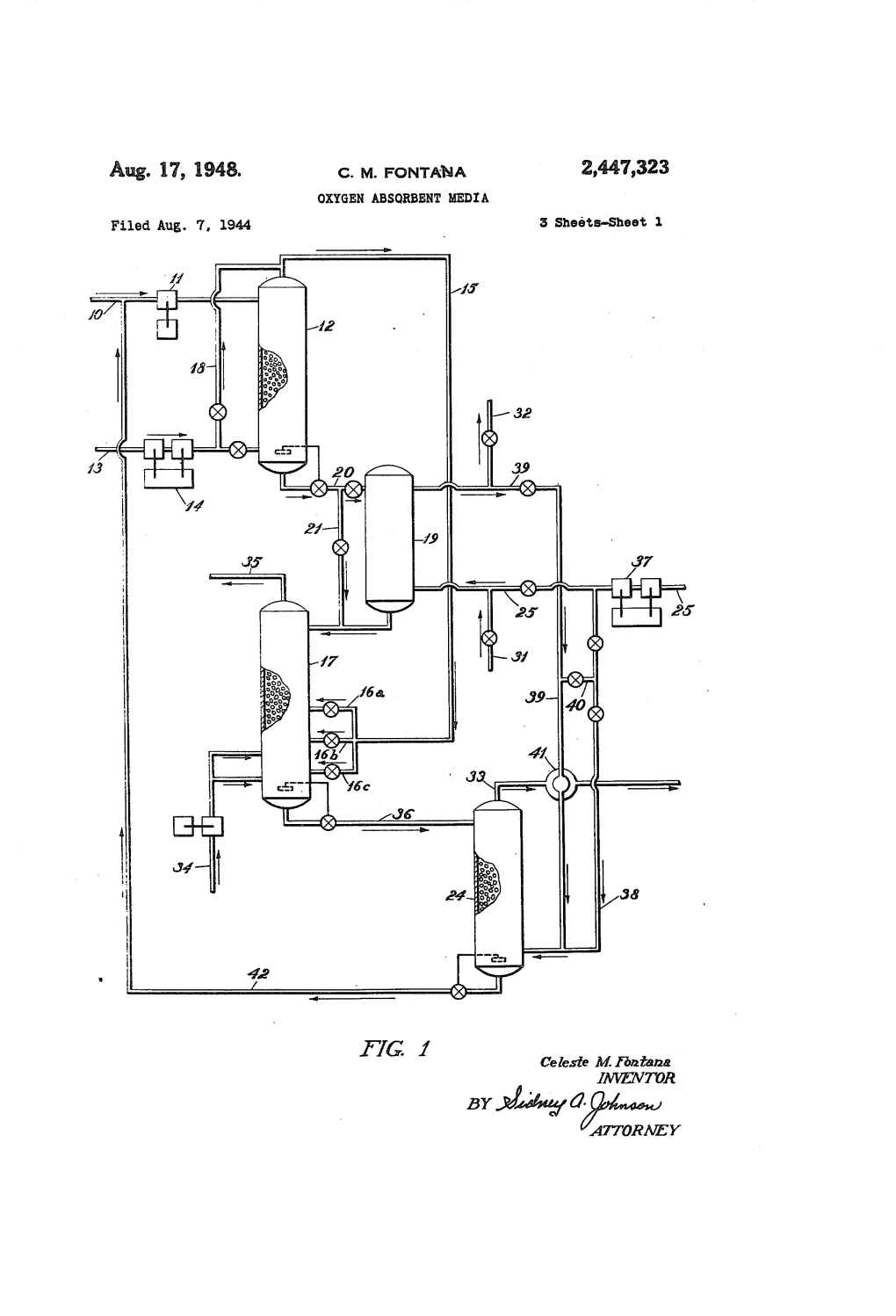
Aug. 17, 1948. C
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Measurements and Modeling of Gas Hydrates Formation in Inhibited Systems: High Pressure, High Salinity and Mixture of Inhibitors
MEASUREMENTS AND MODELING OF GAS HYDRATES FORMATION IN INHIBITED SYSTEMS: HIGH PRESSURE, HIGH SALINITY AND MIXTURE OF INHIBITORS by Yue Hu © Copyright by Yue Hu, 2018 All Rights Reserved A thesis submitted to the Faculty and the Board of Trustees of the Colorado School of Mines in partial fulfillment of the requirements for the degree of Doctor of Philosophy (Chemical Engineering). Golden, Colorado Date Signed: Yue Hu Signed: Dr. Amadeu K. Sum Thesis Advisor Golden, Colorado Date Signed: Dr. Jennifer Wilcox Associate Professor and Interim Head Department of Chemical and Biological Engineering ii ABSTRACT As energy demands continuously increase, oil and gas fields delve into ultra-deep water, which leads to severe operating conditions in terms of pressure, temperature, and water salinity. These conditions pose significant flow assurance challenges, especially gas hydrate formation and scale deposition. Reliable prediction of hydrate phase equilibrium at extreme conditions, in terms of high salinity and high pressure, is necessary for development and operations in ultra-deep water oil and gas production. However, according to the literature review, no open literature studies exist for the hydrate phase equilibria in brine systems above 69 MPa (10,000 psia) due to the challenges associated with experimental designs, safety issues and pitting corrosion problems. As a result, current hydrate prediction tools commonly used are not fully benchmarked and become unreliable at the extreme conditions of very high pressure and high salinity. In this study, experimental data on methane hydrate phase equilibria containing electrolytes, sodium chlo- ride (NaCl), potassium chloride (KCl), and ammonium chloride (NH4Cl) were measured for concentrations up to about 10 wt% at pressure below 10.3 MPa through both isochoric and differential scanning calorimetry (DSC) method with stepwise heating. -

Vaccine Excipient Table
Vaccine Excipient Summary Excipients Included in U.S. Vaccines, by Vaccine In addition to weakened or killed disease antigens (viruses or bacteria), vaccines contain very small amounts of other ingredients – excipients. Some excipients are added to a vaccine for a specific purpose. These include: Preservatives, to prevent contamination. For example, thimerosal. Adjuvants, to help stimulate a stronger immune response. For example, aluminum salts. Stabilizers, to keep the vaccine potent during transportation and storage. For example, sugars or gelatin. Others are residual trace amounts of materials that were used during the manufacturing process and removed. These can include: Cell culture materials, used to grow the vaccine antigens. For example, egg protein, various culture media. Inactivating ingredients, used to kill viruses or inactivate toxins. For example, formaldehyde. Antibiotics, used to prevent contamination by bacteria. For example, neomycin. The following table lists substances, other than active ingredients (i.e., antigens), shown in the manufacturers’ package insert (PI) as being contained in the final formulation of each vaccine. Note: Substances used in the manufacture of a vaccine but not listed as contained in the final product (e.g., culture media) can be found in each PI, but are not shown on this table. Each PI, which can be found on the FDA’s website (see below) contains a description of that vaccine’s manufacturing process, including the amount and purpose of each substance. In most PIs, this information is found -

Potassium Chloride Solution, 0.075M KCL
Potassium Chloride Solution, 0.075M KCL 1. Identification Product Name: Potassium Chloride Solution, 0.075M KCL Item #: MA0507001 Web SDS: N/A Synonyms: N/A Recommended Use: Laboratory Reagent Restrictions on Use: Any use other than recommended Manufacturer: In Case of Emergency: BBC Biochemical Chemtrec US 1-800-424-9300 409 Eleanor Lane, Chemtrec International 703-527-3887 Mount Vernon, WA 98273 1-800-635-4477 2. Hazards Identification OSHA Hazard Classification(s): No OSHA Hazard Classifications Applicable Signal Word: N/A Hazard Statement(s): N/A Pictogram(s): N/A Precautionary Statement(s): Prevention:N/A Response:N/A Storage:N/A Disposal:N/A Descriptions of Hazards not otherwise classified: N/A Percent of mixture with unknown acute toxicity: N/A 3. Composition and Information on Ingredients Chemical Name Common Name CAS # Concentration % Water 7732-18-5 Balance Potassium Chloride 7447-40-7 0.075M 4. First Aid Measures Eye Contact: If in eyes: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. Skin Contact: If on skin (or hair): Take off immediately all contaminated clothing and wash before reuse. Wash with plenty of water. If skin irritation occurs: Get medical advice/attention. Inhalation: Remove to fresh air; give artificial respiration if breathing has stopped. Get medical advice/attention if you feel unwell. Ingestion: Do not induce vomiting. If vomiting occurs spontaneously, keep head below hips to prevent aspiration of liquid into the lungs. Get medical attention. Symptoms: Irritation eyes, nose, throat; headache, dizziness Recommendations for immediate medical care/special treatment: Get medical advice/attention if you feel unwell. -

Did You Know That Vaccines Now Contain Glyphosate in Independent
Did you know that vaccines now contain Glyphosate in independent laboratory testing? This is why we NEED EXEMPTIONS, because the data supplied by the pharmaceutical industry is falsified. This list was made before the glyphosate discovery. It is in the flu virus as well. Polysorbate 80 allows the toxins to cross the blood brain barrier. https://www.momsacrossamerica.com/fda_hides_information_on_glyphosate_in_vaccines Chris Wrinn Milford, CT 06460 Would you put these in your babies or children knowing they have never been tested for safety? The Toxic Ingredients in Innoculations https://www.westonaprice.org/health-topics/vaccinations/adjuvants-in-vaccines/ Most people who vaccinate their children do not realize the kind of ingredients contained in vaccines—and even if they do know, they may not fully understand what that particular ingredient does or what it means. This article is written to help those individuals better understand what they are injecting into the bodies of their loved ones. What prompted me to put this list together was the staggering number of people reporting adverse reactions to vaccines. I wanted to know why so many children experience many of the same reactions. What I found was that many of the adverse reactions fit into many of the side effects of many ingredients contained in vaccines. Please educate before you vaccinate! Don’t wait for something bad to happen before you begin researching vaccines. INGREDIENTS OF COMMON VACCINES DTaP (Infanrix) (Diphtheria, Tetanus, Pertussis) Aluminum Hydroxide, Bovine Extract, -

Potassium Chloride 1311
ASHP INJECTABLE DRUG INFORMATION Potassium CHLORIDE 1311 Potassium Chloride AHFS 40:12 Products chloride injection thoroughly with the infusion solution before administration. The usual maximum concentration is 40 mEq/L. Potassium chloride is available as concentrated solutions of 1.5 and Extravasation should be avoided.1(5/06) 4 2 mEq/mL in 10-, 20-, 30-, and 40-mEq sizes in water for injection in ampuls, vials, and syringes. It is also available in a 30-mL (60-mEq) Great care is required when adding potassium chloride to multiple-dose vial containing methylparaben 0.05% and propyl- infusion solutions, whether in flexible plastic containers or paraben 0.005% as preservatives and 250-mL pharmacy bulk pack- in rigid bottles. Adding potassium chloride to running infu- ages. The pH may have been adjusted with hydrochloric acid and sion solutions hanging in the use position, especially in flex- if necessary potassium hydroxide during manufacture. The concen- ible containers, has resulted in the pooling of potassium chlo- trated solutions must be diluted for use.1(5/06) ride and a resultant high-concentration bolus of the drug being administered to patients, with serious and even fatal conse- Potassium chloride is also available premixed in infusion 4 quences. Attempts to mix adequately the potassium chloride solutions in concentrations of 10, 20, 30, and 40 mEq/L. in flexible containers by squeezing the container in the hanging pH position were unsuccessful. It is recommended that drugs be The pH is usually near 4.6 with a range from 4 to 8.1(5/06) 17 admixed with solutions in flexible containers when positioned with the injection arm of the container uppermost. -

United States Patent Office Patented Dec
3,357,800 United States Patent Office Patented Dec. 12, 1967 2 3,357,800 In addition, the methanol-to-water weight ratio should PURFICATION OF MAGNES JRA CHELORDE not exceed about 8.75. Other solvent-to-solid weight ratios WTH ALCOHOL together with other solvent-to-water ratios, depending on Remigius A. Gaska, Midland, Mich., assignor to The Dow the temperature, the hydrate to be recovered, and the Chemical Company, Midland, Mich, a corporation of Solvent employed may also be used as determined by one Delaware skilled in the art. No Drawing. Fied Nov. 26, 1963, Ser. No. 326,220 Separation and recovery of magnesium chloride by the 7 Claims. (C. 23-297) present invention is accomplished, preferably with active stirring or agitation, at an apparent pH of about 5 or This invention relates to the separation and recovery 10 below, thereby avoiding formation of solid alcoholates, of magnesium chloride from solid mixtures of magnesium and at a temperature within the range of from about 0. chloride and alkali metal halides and more particularly, to about 50° C., preferably between 20 and 35° C. relates to a novel process for recovering the hydrates of Though higher temperatures may be employed in sepa said chloride in a highly purified form. rating magnesium chloride, to do so is inefficient inas Magnesium chloride is ordinarily recovered from aque 15 much as it necessitates the use of pressure equipment. ous solutions containing magnesium chloride and pri- . Conveniently, the present process may readily be carried marily other dissolved alkali metal and alkaline earth out at the normal ambient temperature and pressure. -

Engineering Alcohol Tolerance in Yeast
Engineering alcohol tolerance in yeast The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Lam, F. H., A. Ghaderi, G. R. Fink, and G. Stephanopoulos. “Engineering Alcohol Tolerance in Yeast.” Science 346, no. 6205 (October 2, 2014): 71–75. As Published http://dx.doi.org/10.1126/science.1257859 Publisher American Association for the Advancement of Science (AAAS) Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/99498 Terms of Use Article is made available in accordance with the publisher's policy and may be subject to US copyright law. Please refer to the publisher's site for terms of use. Title: Engineering alcohol tolerance in yeast Authors: Felix H. Lam1,2, Adel Ghaderi1, Gerald R. Fink2*, Gregory Stephanopoulos1* Affiliations: 1 Department of Chemical Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts, USA 2 Whitehead Institute for Biomedical Research, Cambridge, Massachusetts, USA * Correspondence to: G.R.F. ([email protected]) or G.S. ([email protected]) Abstract: Ethanol toxicity in yeast Saccharomyces cerevisiae limits titer and productivity in the industrial production of transportation bioethanol. We show that strengthening the opposing potassium and proton electrochemical membrane gradients is a mechanism that enhances general resistance to multiple alcohols. Elevation of extracellular potassium and pH physically bolster these gradients, increasing tolerance to higher alcohols and ethanol fermentation in commercial and laboratory strains (including a xylose-fermenting strain) under industrial-like conditions. Production per cell remains largely unchanged with improvements deriving from heightened population viability. Likewise, up-regulation of the potassium and proton pumps in the laboratory strain enhances performance to levels exceeding industrial strains. -

Water-Glycine-Potassium Chloride at 25 ?C from Vapor Pressure
JOURNAL OF RESEARCH of the National Bureau of Standards-A. Physics and Chemistry Vol. 69A, No.2, March- April, 1965 Thermodynamics of the Ternary System: Water-Glycine Potassium Chloride at 25 °C From Vapor Pressure Measurements V. E. Bower and R. A. Robinson (I\ovcmbcI' 18, 1964) T he thcrmodynam ic p ropcrt ies of the tC ]'Jl a ry system : water-glycine-potassium chlori de at 25 DC have beell evaluatecl by means of isop iestic vapor p ressul'C' measurements. The effect of added glycin c on t hc uct i\'ity coe ffi cient of p otassiu m chlorirk has been calculatNI an d the effect of added potassium chloride on tiot' acti\' ity coefficien t of glycine. T he "" ria tion of t he activity coefficient of potassium chlori de has bl'l' n sturlit'cl p rl'viou,ly by Roi)l'rts an cl K ir kll'oocl, using a n ent irely cliffc're nt m cihod- tlw Ill l'llSU!'('1l1t'nt of thl' emf of suitable concentration ccli s. T he agreellwn t bet\l'cen illest' in riept' nrle ll t m casurt'mcnts is shOII'n to bl' cxcdlcnt \I' ithin t.he concc ntration rangl'S common to both l'X 1Wl'i nwnts. 1. Introduction Roberts alld Kirkwood [6] meas Llred the emf of the cell: Ag, AgCl I K Cl ( In c) I glycine (m/J) , K(,l The ternary system: water-sucrose-m a nnitol hets (m e) I AgCl, Ag and calcul ated therefrom the effect been innstigated [1]1 by means of isopiesti c vapor of glycine on the activity coeffi cient of potassium pressure measurements. -

Potassium Chloride Extended-Release Tablets
Potassium Chloride Extended-Release Tablets Type of Posting Revision Bulletin Posting Date 20–Aug–2019 Official Date 21–Aug–2019 Expert Committee Chemical Medicines Monographs 5 Reason for Revision Compliance In accordance with the Rules and Procedures of the 2015–2020 Council of Experts, the Chemical Medicines Monographs 5 Expert Committee has revised the Potassium Chloride Extended-Release Tablets monograph. The purpose for the revision is to add Dissolution Test 3 to accommodate FDA- approved drug products with different dissolution conditions and tolerances than the existing dissolution tests. • Dissolution Test 3 was validated using a Dionex IonPac CS12A brand of L106 column from ThermoFisher. The typical retention time for potassium is about 5 min. USP Reference Standards information has been incorporated to support the inclusion of Dissolution Test 3, which includes USP Potassium Chloride RS. The Potassium Chloride Extended-Release Tablets Revision Bulletin supersedes the currently official monograph. Should you have any questions, please contact Ren-Hwa Yeh, Senior Scientific Liaison (301-998-6818 or [email protected]). C207670-M67390-CHM52015, rev 00 20190820 Revision Bulletin Potassium 1 Official August 21, 2019 powdered Tablets, prepared as follows. Transfer an Potassium Chloride Extended-Release appropriate amount of the powder, equivalent to about Tablets 5–6 Tablets, to a suitable volumetric flask, add 10% of the final flask volume of acetone, and sonicate for 45 DEFINITION min with intermittent shaking. Add 80% of the final flask Potassium Chloride Extended-Release Tablets contain NLT volume of water and sonicate for 45 min with 90.0% and NMT 110.0% of the labeled amount of intermittent shaking. -

Hazardous Chemicals in Synthetic Turf Materials and Their Bioaccessibility in Digestive fluids
Journal of Exposure Science and Environmental Epidemiology (2008) 18, 600–607 r 2008 Nature Publishing Group All rights reserved 1559-0631/08/$30.00 www.nature.com/jes Hazardous chemicals in synthetic turf materials and their bioaccessibility in digestive fluids JUNFENG (JIM) ZHANGa, IN-KYU HANa,b,LINZHANGa AND WILLIAM CRAINc aSchool of Public Health, University of Medicine and Dentistry of New Jersey, 683 Hoes Lane West, Piscataway, New Jersey, USA bJohns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA cThe City College of New York, New York, New York, USA Many synthetic turf fields consist of not only artificial grass but also rubber granules that are used as infill. The public concerns about toxic chemicals possibly contained in either artificial (polyethylene) grass fibers or rubber granules have been escalating but are based on very limited information available to date. The aim of this research was to obtain data that will help assess potential health risks associated with chemical exposure. In this small- scale study, we collected seven samples of rubber granules and one sample of artificial grass fiber from synthetic turf fields at different ages of the fields. We analyzed these samples to determine the contents (maximum concentrations) of polycyclic aromatic hydrocarbons (PAHs) and several metals (Zn, Cr, As, Cd, and Pb). We also analyzed these samples to determine their bioaccessible fractions of PAHs and metals in synthetic digestive fluids including saliva, gastric fluid, and intestinal fluid through a laboratory simulation technique. Our findings include: (1) rubber granules often, especially when the synthetic turf fields were newer, contained PAHs at levels above health-based soil standards. -

The Elements.Pdf
A Periodic Table of the Elements at Los Alamos National Laboratory Los Alamos National Laboratory's Chemistry Division Presents Periodic Table of the Elements A Resource for Elementary, Middle School, and High School Students Click an element for more information: Group** Period 1 18 IA VIIIA 1A 8A 1 2 13 14 15 16 17 2 1 H IIA IIIA IVA VA VIAVIIA He 1.008 2A 3A 4A 5A 6A 7A 4.003 3 4 5 6 7 8 9 10 2 Li Be B C N O F Ne 6.941 9.012 10.81 12.01 14.01 16.00 19.00 20.18 11 12 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 3 Na Mg IIIB IVB VB VIB VIIB ------- VIII IB IIB Al Si P S Cl Ar 22.99 24.31 3B 4B 5B 6B 7B ------- 1B 2B 26.98 28.09 30.97 32.07 35.45 39.95 ------- 8 ------- 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 4 K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr 39.10 40.08 44.96 47.88 50.94 52.00 54.94 55.85 58.47 58.69 63.55 65.39 69.72 72.59 74.92 78.96 79.90 83.80 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 5 Rb Sr Y Zr NbMo Tc Ru Rh PdAgCd In Sn Sb Te I Xe 85.47 87.62 88.91 91.22 92.91 95.94 (98) 101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.9 131.3 55 56 57 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 6 Cs Ba La* Hf Ta W Re Os Ir Pt AuHg Tl Pb Bi Po At Rn 132.9 137.3 138.9 178.5 180.9 183.9 186.2 190.2 190.2 195.1 197.0 200.5 204.4 207.2 209.0 (210) (210) (222) 87 88 89 104 105 106 107 108 109 110 111 112 114 116 118 7 Fr Ra Ac~RfDb Sg Bh Hs Mt --- --- --- --- --- --- (223) (226) (227) (257) (260) (263) (262) (265) (266) () () () () () () http://pearl1.lanl.gov/periodic/ (1 of 3) [5/17/2001 4:06:20 PM] A Periodic Table of the Elements at Los Alamos National Laboratory 58 59 60 61 62 63 64 65 66 67 68 69 70 71 Lanthanide Series* Ce Pr NdPmSm Eu Gd TbDyHo Er TmYbLu 140.1 140.9 144.2 (147) 150.4 152.0 157.3 158.9 162.5 164.9 167.3 168.9 173.0 175.0 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Actinide Series~ Th Pa U Np Pu AmCmBk Cf Es FmMdNo Lr 232.0 (231) (238) (237) (242) (243) (247) (247) (249) (254) (253) (256) (254) (257) ** Groups are noted by 3 notation conventions. -

Replacement of Potassium Ions by Ammonium Ions in Potassium-Limited Chemostat Cultures of Candida Utilis NCYC
FEMS MicrobiologyLetters 107 (1993) 1-4 © 1993 Federation of European MicrobiologicalSocieties 0378-1097/93/$06.00 Published by Elsevier FEMSLE 05264 Replacement of potassium ions by ammonium ions in potassium-limited chemostat cultures Downloaded from https://academic.oup.com/femsle/article/107/1/1/627950 by guest on 27 September 2021 of Candida utilis NCYC 321 Ed T. Buurman 1, Paul T.D. Herman, M. Joost Teixeira de Mattos and Oense M. Neijssel Department of Microbiology, Biotechnology Centre, Unicersity of Amsterdam, Amsterdam, Netherlands (Received 20 October 1992; revision received and accepted 12 November 1992) Abstract: Potassium-limited cultures of Candida utilis grown with ammonium chloride as the sole nitrogen source attained a higher dry weight than similar cultures grown with sodium nitrate as the sole nitrogen source. This increase depended on the extracellular ammonia concentration and was not due to accumulation of storage polymers. We conclude that in this yeast ammonium ions can fulfill to some extent the physiological role of potassium ions and that the intracellular concentration of ammonium ions is predominantly determined by the ammonia concentration in the culture medium. Key words: Candida utilis; Potassium; Ammonium; pH; Chemostat culture Introduction gests that ammonium ions can contribute signifi- cantly to the osmotic pressure of the cytoplasm. It Prokaryotes require a high intracellular con- has been proposed that a high intracellular am- centration of potassium ions in order to activate monium ion concentration could be built up, be- the ribosomes [1] and to maintain the osmotic cause the ammonia molecule is extremely perme- pressure of the cytoplasm [2]. Recently it has able through the bacterial membrane.