Full-Text (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

The Reactions of Limmu Oromo of Western Ethiopia During and After the Italian Occupation (1936-41)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by International Institute for Science, Technology and Education (IISTE): E-Journals Historical Research Letter www.iiste.org ISSN 2224-3178 (Paper) ISSN 2225-0964 (Online) Vol.47, 2018 The Reactions of Limmu Oromo of Western Ethiopia During and After the Italian Occupation (1936-41) Fekede Sileshi Fufa 1 Ketebo Abdiyo(PhD) 2 1.College of Social Science Department of History, Sebeta Special Needs College of Teacher’s Education 2.Jimma University Abstract In this paper, it has been attempted to highlight the features of the five years administration system and the continuity and changes that took place during the Italy administration in the area. During the Italian Occupation, Limmu was the administrative center of the Italians for the Oromo of Western Anger River. Surprisingly, Limmu Oromo during the Italian occupation (1936-41) fought both the Italians and the naftañä settlers. According to genuine sources, during Italian Occupation (1936-1941) the naftañä-gabär system was removed in the study area. Accordingly, the most important event of this period was that the Limmu Oromo were librated from the naftañä rule by their leader, Fitawurari Duguma Jaldeso. Relatively speaking, according to many written materials and oral sources, the Italian administration was better than the former administration system in Limmu. The Italian Occupation was a land mark in the history of the gabärs in the surrounding region in general and that of Limmu in particular. Soon after they stationed at Ayana (the capital of Gidda-Kiramu Woreda), the Italian military station for the areas between the Abay and Angar, Italians successfully abolished the gabär system in the area. -

Vulnerability Analysis of Smallholder
Tessema and Simane Ecological Processes (2019) 8:5 https://doi.org/10.1186/s13717-019-0159-7 RESEARCH Open Access Vulnerability analysis of smallholder farmers to climate variability and change: an agro- ecological system-based approach in the Fincha’a sub-basin of the upper Blue Nile Basin of Ethiopia Israel Tessema1,2* and Belay Simane1 Abstract Background: Ethiopia is frequently cited as a country that is highly vulnerable to climate variability and change. The country’s high vulnerability arises mostly from climate-sensitive agricultural sector that suffers a lot from risks associated with rainfall variability. The vulnerability factors (exposure, sensitivity, and adaptive capacity) of the agricultural livelihoods to climate variability and change differ across agro-ecological systems (AESs). Therefore, the aim of this study was to analyze AES-specific vulnerability of smallholder farmers to climate variability and change in the Fincha’a sub-basin. We surveyed 380 respondents from 4 AESs (highland, midland, wetland, and lowland) randomly selected. Furthermore, focus group discussion and key informant interviews were also performed to supplement and substantiate the quantitative data. Livelihood vulnerability index was employed to analyze the levels of smallholders’ agriculture vulnerability to climate variability and change. Data on socioeconomic and biophysical attribute were collected and combined into the indices and vulnerability score was calculated for each agro-ecological system. Results: Considerable variation was observed across the agro-ecological systems in profile, indicator, and the three livelihood vulnerability indices-Intergovernmental Panel on Climate Change dimensions (exposure, sensitivity, and adaptive capacity) of vulnerability. The lowland AES exhibited higher exposure, low adaptive capacity, and high vulnerability, while the midland AES demonstrated lower exposure, higher adaptive capacity, and lower vulnerability. -

Analysis of Productivity and Efficiency of Maize Production in Gardega-Jarte District of Ethiopia
World Journal of Agricultural Sciences 15 (3): 180-193, 2019 ISSN 1817-3047 © IDOSI Publications, 2019 DOI: 10.5829/idosi.wjas.2019.180.193 Analysis of Productivity and Efficiency of Maize Production in Gardega-Jarte District of Ethiopia 12Hika Wana and Afsaw Lemessa 1Wollega University, Department of Agricultural Economics, P.O. Box, 395, Nekempt, Ethiopia 2Gardega-Jarte, Agricultural Office, P.O. Box, Shambu, Ethiopia Abstract: The aim of the study was to estimate technical efficiency of smallholder farmers in maize production in case of Jardega Jarte districts with specific objectives to estimate the level of technical efficiency and to identify factors affecting technical efficiency in the study area. The study used cross-sectional data and the data were collected from sample representative respondents of 168 randomly selected farm households. Cobb-Douglas production function and the Stochastic Frontier Model were used to identify factors influencing productivity and efficiency. The hypotheses tests confirm that, the adequacy of Cobb-Douglas the appropriateness of using SFA the joint statistical significance of inefficiency effects; the appropriateness of using Half- normal and Exponential distribution for one sided error; and nature of the stochastic production function. The maximum likelihood parameter estimates showed that all input variables have positive and significant effect on production. The estimated Cob Douglas production function revealed that all inputs labor in hour, maize cultivated land, Dap, Urea, Seed, oxen have positive -

Oromia Region Administrative Map(As of 27 March 2013)
ETHIOPIA: Oromia Region Administrative Map (as of 27 March 2013) Amhara Gundo Meskel ! Amuru Dera Kelo ! Agemsa BENISHANGUL ! Jangir Ibantu ! ! Filikilik Hidabu GUMUZ Kiremu ! ! Wara AMHARA Haro ! Obera Jarte Gosha Dire ! ! Abote ! Tsiyon Jars!o ! Ejere Limu Ayana ! Kiremu Alibo ! Jardega Hose Tulu Miki Haro ! ! Kokofe Ababo Mana Mendi ! Gebre ! Gida ! Guracha ! ! Degem AFAR ! Gelila SomHbo oro Abay ! ! Sibu Kiltu Kewo Kere ! Biriti Degem DIRE DAWA Ayana ! ! Fiche Benguwa Chomen Dobi Abuna Ali ! K! ara ! Kuyu Debre Tsige ! Toba Guduru Dedu ! Doro ! ! Achane G/Be!ret Minare Debre ! Mendida Shambu Daleti ! Libanos Weberi Abe Chulute! Jemo ! Abichuna Kombolcha West Limu Hor!o ! Meta Yaya Gota Dongoro Kombolcha Ginde Kachisi Lefo ! Muke Turi Melka Chinaksen ! Gne'a ! N!ejo Fincha!-a Kembolcha R!obi ! Adda Gulele Rafu Jarso ! ! ! Wuchale ! Nopa ! Beret Mekoda Muger ! ! Wellega Nejo ! Goro Kulubi ! ! Funyan Debeka Boji Shikute Berga Jida ! Kombolcha Kober Guto Guduru ! !Duber Water Kersa Haro Jarso ! ! Debra ! ! Bira Gudetu ! Bila Seyo Chobi Kembibit Gutu Che!lenko ! ! Welenkombi Gorfo ! ! Begi Jarso Dirmeji Gida Bila Jimma ! Ketket Mulo ! Kersa Maya Bila Gola ! ! ! Sheno ! Kobo Alem Kondole ! ! Bicho ! Deder Gursum Muklemi Hena Sibu ! Chancho Wenoda ! Mieso Doba Kurfa Maya Beg!i Deboko ! Rare Mida ! Goja Shino Inchini Sululta Aleltu Babile Jimma Mulo ! Meta Guliso Golo Sire Hunde! Deder Chele ! Tobi Lalo ! Mekenejo Bitile ! Kegn Aleltu ! Tulo ! Harawacha ! ! ! ! Rob G! obu Genete ! Ifata Jeldu Lafto Girawa ! Gawo Inango ! Sendafa Mieso Hirna -

190327 Oromia Region Agric S
ETHIOPIA: AGRICULTURE SECTOR HRP OROMIA REGION MONTHLY DASHBOARD - March 2019 The devastating impact on agriculture following consecutive years of drought in Ethiopia is undisputed. While forecasts for 2019 indicate a probability of normal to above normal rain in most parts of Ethiopia, in east, south and southeastern regions, the upcoming rainy season (March to June) is forecast- KEY FIGURES ed to be average or below average. In areas where normal to above normal rains are expected, recovery will not be spontaneous, as previous OVERVIEW HOUSEHOLDS REACHED drought-affected households are likely to require sustained humanitarian assistance as a result of exhausted coping mechanisms. HOUSEHOLDS IN NEED Humanitarian assistance for IDPs and IDP returnees is largely dependent on IDPs’ access to land and the livelihood assets they have been able to 1.15 million maintain during displacement. Emergency feed and animal health interventions are needed to reduce the burden on the resources of the host 0.0m 0% communities and prevent the spread of diseases, especially for animals displaced across regional borders. Where appropriate, land will be availed and crop seeds, farming tools, and training will be provided to support IDP and returning households to improve their food security and reduce the burden HOUSEHOLDS TARGETED on host Communities. 658,428 IDP HOUSEHOLDS TARGETED N_Shewa 0m 0% Horo 64,195 Guduru Chinaksen Guto W_Wellega Gida Meta Sasiga Finfine Doba Gursum AGRICULTURE LIVESTOCK E_Wellega Mieso Crop Seeds &Tools W_Shewa Special Girawa -

ETHIOPIA - National Hot Spot Map 31 May 2010
ETHIOPIA - National Hot Spot Map 31 May 2010 R Legend Eritrea E Tigray R egion !ª D 450 ho uses burned do wn d ue to th e re ce nt International Boundary !ª !ª Ahferom Sudan Tahtay Erob fire incid ent in Keft a hum era woreda. I nhabitan ts Laelay Ahferom !ª Regional Boundary > Mereb Leke " !ª S are repo rted to be lef t out o f sh elter; UNI CEF !ª Adiyabo Adiyabo Gulomekeda W W W 7 Dalul E !Ò Laelay togethe r w ith the regiona l g ove rnm ent is Zonal Boundary North Western A Kafta Humera Maychew Eastern !ª sup portin g the victim s with provision o f wate r Measle Cas es Woreda Boundary Central and oth er imm ediate n eeds Measles co ntinues to b e re ported > Western Berahle with new four cases in Arada Zone 2 Lakes WBN BN Tsel emt !A !ª A! Sub-city,Ad dis Ababa ; and one Addi Arekay> W b Afa r Region N b Afdera Military Operation BeyedaB Ab Ala ! case in Ahfe rom woreda, Tig ray > > bb The re a re d isplaced pe ople from fo ur A Debark > > b o N W b B N Abergele Erebtoi B N W Southern keb eles of Mille and also five kebeles B N Janam ora Moegale Bidu Dabat Wag HiomraW B of Da llol woreda s (400 0 persons) a ff ected Hot Spot Areas AWD C ases N N N > N > B B W Sahl a B W > B N W Raya A zebo due to flo oding from Awash rive r an d ru n Since t he beg in nin g of th e year, Wegera B N No Data/No Humanitarian Concern > Ziquala Sekota B a total of 967 cases of AWD w ith East bb BN > Teru > off fro m Tigray highlands, respective ly. -

Somali Region
Food Supply Prospects FOR THE SECOND HALF OF YEAR 2013 ______________________________________________________________________________ Disaster Risk Management and Food Security Sector (DRMFSS) Ministry of Agriculture (MoA) September, 2013 Addis Ababa, Ethiopia TABLE OF CONTENTS GLOSSARY OF LOCAL NAMES .................................................................. 1 ACRONYMS ............................................................................................. 2 EXCUTIVE SUMMARY .............................................................................. 3 INTRODUCTION ....................................................................................... 7 REGIONAL SUMMARY OF FOOD SUPPLY PROSPECT ............................. 11 SOMALI .............................................................................................. 11 OROMIA ............................................................................................. 16 TIGRAY ............................................................................................... 22 AMHARA ............................................................................................ 25 AFAR .................................................................................................. 28 SNNP .................................................................................................. 32 Annex – 1: NEEDY POPULATION AND FOOD REQUIREMENT BY WOREDA (Second half of 2013) ............................................................................ 35 0 | P a g e GLOSSARY -

Original Research Original Research
DOI: http://dx.doi.org/10.4314/star.v4i2.28 ISSN: 2226-7522(Print) and 2305 -3372 (Online) Science, Technology and Arts Research Journal Sci. Technol. Arts Res. J., April-June 201 5, 4 (2): 215-221 Journal Homepage: http://www.starjournal.org/ Original Research Assessment on Dairy Production System and its Constraints in Horoguduru Wollega Zone, Western Ethiopia Belay Beyene 1*, Demissu Hundie 1 and Geleta Gobena 2 1Faculty of Agriculture, Department of Animal ScienceScience,, Wollega University, Shambu Campus, Shambu, Ethiopia 2Horo Guduru Animal Production and Research Center, Wollega University, Guduru, Ethiopia Abstract Article Information A cross sectional study was conducted to assess dairy production system and to identify its Article History: common constraints in Abay Choman and Jimma Ganati Districts of Horro Guduru Wollega Received : 12-04-2015 zone. Multistage sampling technique was used to determine sample size and Districts, peasant associations, villages and dairy owners were sampled sequentially. 63.13% of the Revised : 13-06-2015 respondents had formal education; out of which 40.22% attended only elementary Accepted : 18-06-2015 education, 8.94% attended high School, 7.26% diploma graduate and 6.70% were degree graduates. The means ± SD of cattle hol ding was 8.04 ± 6.72 out of which averagely 3 Keywords : animals were cows. Of the total of 546 dairy cows, only 9 (1.65%) and 5 (0.92%) were Dairy product Holstein and Jersey cross bred cows. Housing system of the study area was mainly Horro Guduru traditional and unimproved where 91.62% of dairy cows pass the night in earthen floor housing of which 77.09% were not shaded. -
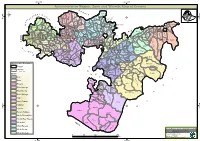
Administrative Region, Zone and Woreda Map of Oromia a M Tigray a Afar M H U Amhara a Uz N M
35°0'0"E 40°0'0"E Administrative Region, Zone and Woreda Map of Oromia A m Tigray A Afar m h u Amhara a uz N m Dera u N u u G " / m r B u l t Dire Dawa " r a e 0 g G n Hareri 0 ' r u u Addis Ababa ' n i H a 0 Gambela m s Somali 0 ° b a K Oromia Ü a I ° o A Hidabu 0 u Wara o r a n SNNPR 0 h a b s o a 1 u r Abote r z 1 d Jarte a Jarso a b s a b i m J i i L i b K Jardega e r L S u G i g n o G A a e m e r b r a u / K e t m uyu D b e n i u l u o Abay B M G i Ginde e a r n L e o e D l o Chomen e M K Beret a a Abe r s Chinaksen B H e t h Yaya Abichuna Gne'a r a c Nejo Dongoro t u Kombolcha a o Gulele R W Gudetu Kondole b Jimma Genete ru J u Adda a a Boji Dirmeji a d o Jida Goro Gutu i Jarso t Gu J o Kembibit b a g B d e Berga l Kersa Bila Seyo e i l t S d D e a i l u u r b Gursum G i e M Haro Maya B b u B o Boji Chekorsa a l d Lalo Asabi g Jimma Rare Mida M Aleltu a D G e e i o u e u Kurfa Chele t r i r Mieso m s Kegn r Gobu Seyo Ifata A f o F a S Ayira Guliso e Tulo b u S e G j a e i S n Gawo Kebe h i a r a Bako F o d G a l e i r y E l i Ambo i Chiro Zuria r Wayu e e e i l d Gaji Tibe d lm a a s Diga e Toke n Jimma Horo Zuria s e Dale Wabera n a w Tuka B Haru h e N Gimbichu t Kutaye e Yubdo W B Chwaka C a Goba Koricha a Leka a Gidami Boneya Boshe D M A Dale Sadi l Gemechis J I e Sayo Nole Dulecha lu k Nole Kaba i Tikur Alem o l D Lalo Kile Wama Hagalo o b r Yama Logi Welel Akaki a a a Enchini i Dawo ' b Meko n Gena e U Anchar a Midega Tola h a G Dabo a t t M Babile o Jimma Nunu c W e H l d m i K S i s a Kersana o f Hana Arjo D n Becho A o t -

Ethiopia: Administrative Map (August 2017)
Ethiopia: Administrative map (August 2017) ERITREA National capital P Erob Tahtay Adiyabo Regional capital Gulomekeda Laelay Adiyabo Mereb Leke Ahferom Red Sea Humera Adigrat ! ! Dalul ! Adwa Ganta Afeshum Aksum Saesie Tsaedaemba Shire Indasilase ! Zonal Capital ! North West TigrayTahtay KoraroTahtay Maychew Eastern Tigray Kafta Humera Laelay Maychew Werei Leke TIGRAY Asgede Tsimbila Central Tigray Hawzen Medebay Zana Koneba Naeder Adet Berahile Region boundary Atsbi Wenberta Western Tigray Kelete Awelallo Welkait Kola Temben Tselemti Degua Temben Mekele Zone boundary Tanqua Abergele P Zone 2 (Kilbet Rasu) Tsegede Tselemt Mekele Town Special Enderta Afdera Addi Arekay South East Ab Ala Tsegede Mirab Armacho Beyeda Woreda boundary Debark Erebti SUDAN Hintalo Wejirat Saharti Samre Tach Armacho Abergele Sanja ! Dabat Janamora Megale Bidu Alaje Sahla Addis Ababa Ziquala Maychew ! Wegera Metema Lay Armacho Wag Himra Endamehoni Raya Azebo North Gondar Gonder ! Sekota Teru Afar Chilga Southern Tigray Gonder City Adm. Yalo East Belesa Ofla West Belesa Kurri Dehana Dembia Gonder Zuria Alamata Gaz Gibla Zone 4 (Fantana Rasu ) Elidar Amhara Gelegu Quara ! Takusa Ebenat Gulina Bugna Awra Libo Kemkem Kobo Gidan Lasta Benishangul Gumuz North Wello AFAR Alfa Zone 1(Awsi Rasu) Debre Tabor Ewa ! Fogera Farta Lay Gayint Semera Meket Guba Lafto DPubti DJIBOUTI Jawi South Gondar Dire Dawa Semen Achefer East Esite Chifra Bahir Dar Wadla Delanta Habru Asayita P Tach Gayint ! Bahir Dar City Adm. Aysaita Guba AMHARA Dera Ambasel Debub Achefer Bahirdar Zuria Dawunt Worebabu Gambela Dangura West Esite Gulf of Aden Mecha Adaa'r Mile Pawe Special Simada Thehulederie Kutaber Dangila Yilmana Densa Afambo Mekdela Tenta Awi Dessie Bati Hulet Ej Enese ! Hareri Sayint Dessie City Adm. -

OROMIA REGION - Regional 3W Map 07 December 2010
OROMIA REGION - Regional 3W Map 07 December 2010 CRS I SC-UK V Legend W Amhara S d S Farm Africa R S i E R C Benishangul R A V E CRS CARE MfM C GOAL C P n R W S o i I International Boundary SC-Denmark A , t Gumuz Afar C L c C P K Action Aid C A CARE Welthungerhilfe A CRS S U I M - I ! O C C IMC S S G S a CARE A WVE S Regional Boundary , SC-Denmark R R c m i SC-Denmark Dera C C a S r R f u u u c i C U E A Action Aid t r S m r n u f GOAL e R m R a r A m E IMC r i E C A b a I L L a R R Zonal Boundary K CARE ! f F o C Action Aid H s A a Hidabu Abote m r ! A A g a A r J x a a e A a C r Christian Aid C O d a C d r m O a r b O IMC Action Aid i W a M CA RE o J e I a G F G G G u e ! g L t n b r b e i a i i n i J o E S m u D a d Farm Africa Gerar Jarso R CARE Woreda Boundary E IMC u e p Kuyu E a ! Kiltu Kara m A i n ! o R ! S R C r ! a L Abay Chomen B Debre Libanos o ! Abuna G/Beret A M m M e A H e l o C en a m ks e ina r u Yaya Gulele Abichuna Gne'a C Ch Abe Dongoro ! a h s ! e i t c r a g f a u Nejo t IMC a ! r CRS No Data /No Intervention g E J ! W ! l a x Kombolcha o e R o ! d V e O b B i o ! ! s Guduru G J ib it Goro Gutu ! a r m b a Gudetu Kondole FHI Ke W a Mercy Corps b a s B u i ! r B Boji Dirmeji o t a Bila Seyo e i ! a r Jimma Genete l J d K L e g t s l Jeldu u E a ! d D ! u F Haro Maya e Guto Gida b M S u A m A o e B e M l R i d ! Boji Chekorsa Jimma Rare S ! D CARE GOAL CRS b a B b u A o Aleltu l e ! Kurfa Chele A g I O u f e e y G i u a S Mieso r Agriculture & Livestock i C r s t ! T a Mida KegnA a M Lalo Asabi im r K a ! ! G S Gobu