Chapter 5.14 Toluene
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review of Market for Octane Enhancers
May 2000 • NREL/SR-580-28193 Review of Market for Octane Enhancers Final Report J.E. Sinor Consultants, Inc. Niwot, Colorado National Renewable Energy Laboratory 1617 Cole Boulevard Golden, Colorado 80401-3393 NREL is a U.S. Department of Energy Laboratory Operated by Midwest Research Institute • Battelle • Bechtel Contract No. DE-AC36-99-GO10337 May 2000 • NREL/SR-580-28193 Review of Market for Octane Enhancers Final Report J.E. Sinor Consultants, Inc. Niwot, Colorado NREL Technical Monitor: K. Ibsen Prepared under Subcontract No. TXE-0-29113-01 National Renewable Energy Laboratory 1617 Cole Boulevard Golden, Colorado 80401-3393 NREL is a U.S. Department of Energy Laboratory Operated by Midwest Research Institute • Battelle • Bechtel Contract No. DE-AC36-99-GO10337 NOTICE This report was prepared as an account of work sponsored by an agency of the United States government. Neither the United States government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States government or any agency thereof. Available electronically at http://www.doe.gov/bridge Available for a processing fee to U.S. -

Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances 2017
INTERNATIONAL NARCOTICS CONTROL BOARD Precursors and chemicals frequently used in the illicit manufacture of narcotic drugs and psychotropic substances 2017 EMBARGO Observe release date: Not to be published or broadcast before Thursday, 1 March 2018, at 1100 hours (CET) UNITED NATIONS CAUTION Reports published by the International Narcotics Control Board in 2017 The Report of the International Narcotics Control Board for 2017 (E/INCB/2017/1) is supplemented by the following reports: Narcotic Drugs: Estimated World Requirements for 2018—Statistics for 2016 (E/INCB/2017/2) Psychotropic Substances: Statistics for 2016—Assessments of Annual Medical and Scientific Requirements for Substances in Schedules II, III and IV of the Convention on Psychotropic Substances of 1971 (E/INCB/2017/3) Precursors and Chemicals Frequently Used in the Illicit Manufacture of Narcotic Drugs and Psychotropic Substances: Report of the International Narcotics Control Board for 2017 on the Implementation of Article 12 of the United Nations Convention against Illicit Traffic in Narcotic Drugs and Psychotropic Substances of 1988 (E/INCB/2017/4) The updated lists of substances under international control, comprising narcotic drugs, psychotropic substances and substances frequently used in the illicit manufacture of narcotic drugs and psychotropic substances, are contained in the latest editions of the annexes to the statistical forms (“Yellow List”, “Green List” and “Red List”), which are also issued by the Board. Contacting the International Narcotics Control Board The secretariat of the Board may be reached at the following address: Vienna International Centre Room E-1339 P.O. Box 500 1400 Vienna Austria In addition, the following may be used to contact the secretariat: Telephone: (+43-1) 26060 Fax: (+43-1) 26060-5867 or 26060-5868 Email: [email protected] The text of the present report is also available on the website of the Board (www.incb.org). -

Including Toluene Derivatives
RSC Advances View Article Online PAPER View Journal | View Issue Catalytic C–H aerobic and oxidant-induced oxidation of alkylbenzenes (including toluene Cite this: RSC Adv., 2020, 10,23543 derivatives) over VO2+ immobilized on core–shell Fe3O4@SiO2 at room temperature in water† Pegah Mohammadpour and Elham Safaei * Direct C–H bond oxidation of organic materials, and producing the necessary oxygenated compounds under mild conditions, has attracted increasing interest. The selective oxidation of various alkylbenzenes 2+ was carried out by means of a new catalyst containing VO species supported on silica-coated Fe3O4 nanoparticles using t-butyl hydroperoxide as an oxidant at room temperature in H2O or solvent-free media. The chemical and structural characterization of the catalyst using several methods such as FTIR spectroscopy, XRD, FETEM, FESEM, SAED, EDX and XPS showed that VO2+ is covalently bonded to the silica surface. High selectivity and excellent conversion of various toluene derivatives, with less reactive Creative Commons Attribution 3.0 Unported Licence. Received 18th April 2020 aliphatic (sp3)C–H bonds, to related benzoic acids were quite noticeable. The aerobic oxygenation Accepted 15th May 2020 reaction of these alkylbenzenes was studied under the same conditions. All the results accompanied by DOI: 10.1039/d0ra03483e sustainability of the inexpensive and simple magnetically separable heterogeneous catalyst proved the rsc.li/rsc-advances important criteria for commercial applications. Introduction industrial communities. Among -

Organocatalytic Asymmetric N-Sulfonyl Amide C-N Bond Activation to Access Axially Chiral Biaryl Amino Acids
ARTICLE https://doi.org/10.1038/s41467-020-14799-8 OPEN Organocatalytic asymmetric N-sulfonyl amide C-N bond activation to access axially chiral biaryl amino acids Guanjie Wang1, Qianqian Shi2, Wanyao Hu1, Tao Chen1, Yingying Guo1, Zhouli Hu1, Minghua Gong1, ✉ ✉ ✉ Jingcheng Guo1, Donghui Wei 2 , Zhenqian Fu 1,3 & Wei Huang1,3 1234567890():,; Amides are among the most fundamental functional groups and essential structural units, widely used in chemistry, biochemistry and material science. Amide synthesis and trans- formations is a topic of continuous interest in organic chemistry. However, direct catalytic asymmetric activation of amide C-N bonds still remains a long-standing challenge due to high stability of amide linkages. Herein, we describe an organocatalytic asymmetric amide C-N bonds cleavage of N-sulfonyl biaryl lactams under mild conditions, developing a general and practical method for atroposelective construction of axially chiral biaryl amino acids. A structurally diverse set of axially chiral biaryl amino acids are obtained in high yields with excellent enantioselectivities. Moreover, a variety of axially chiral unsymmetrical biaryl organocatalysts are efficiently constructed from the resulting axially chiral biaryl amino acids by our present strategy, and show competitive outcomes in asymmetric reactions. 1 Key Laboratory of Flexible Electronics & Institute of Advanced Materials, Jiangsu National Synergetic Innovation Center for Advanced Materials, Nanjing Tech University, 30 South Puzhu Road, Nanjing 211816, China. 2 College -
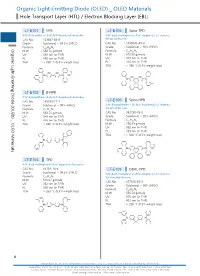
Organic Light-Emitting Diode (OLED) OLED Materials Hole Transport Layer (HTL) / Electron Blocking Layer (EBL)
Organic Light-EmittingOrganic Light-Emitting DiodeHole Transport(OLED) Layer (HTL) / Diode (OLED) _ OLED Materials Electron Blocking Layer (EBL) Organic Light-Emitting Diode (OLED) _ OLED Materials Hole Transport Layer (HTL) / Electron Blocking Layer (EBL) LT-E101 NPB LT-E105 Spiro-TPD N,N'-Bis(naphthalen-1-yl)-N,N'-bis(phenyl)-benzidine N,N'-Bis(3-methylphenyl)-N,N'-bis(phenyl)-2,7-diamino- CAS No. : 123847-85-8 9,9-spirobifluorene Grade : Sublimed, > 99.5% (HPLC) CAS No. : 1033035-83-4 : Grade : Sublimed, > 99% (HPLC) Organic Light-Emitting Diode (OLED) _ OLED Materials Formula C44H32N2 M.W. : 588.74 g/mole Formula : C51H38N2 UV : 339 nm (in THF) M.W. : 678.86 g/mole PL : 450 nm (in THF) UV : 379 nm (in THF) TGA : > 350 °C (0.5% weight loss) PL : 416 nm (in THF) TGA : > 280 °C (0.5% weight loss) N N N N LT-E102 β-NPB N,N'-Bis(naphthalen-2-yl)-N,N'-bis(phenyl)-benzidine Spiro-NPB CAS No. : 139255-17-7 LT-E106 Grade : Sublimed, > 99% (HPLC) N,N'-Bis(naphthalen-1-yl)-N,N'-bis(phenyl)-2,7-diamino- 9,9-spirobifluorene Formula : C44H32N2 M.W. : 588.74 g/mole CAS No. : 932739-76-9 UV : 349 nm (in THF) Grade : Sublimed, > 99% (HPLC) PL : 416 nm (in THF) Formula : C57H38N2 TGA : > 330 °C (0.5% weight loss) M.W. : 750.93 g/mole UV : 380 nm (in THF) PL : 453 nm (in THF) TGA : > 360 °C (0.5% weight loss) N N N N LT-E103 TPD N,N'-Bis(3-methylphenyl)-N,N'-bis(phenyl)-benzidine CAS No. -

Reactions of Benzene Or Alkylbenzenes with Steam Over a Silica-Supported Nickel Catalyst*
54 Reactions of Benzene or Alkylbenzenes with Steam over a Silica-supported Nickel Catalyst* by .Masahiro Saito**, Yoshio Sohda** Michiaki Tokuno** and Yoshiro Morita** Summary: Benzene-steam and alkylbenzenes-steam reactions over a silica-supported nickel catalyst have been studied under atmospheric pressure in a temperature range of 370~430℃. In the reactionof benzene-steam,the methaneyield is lower and the carbon dioxideyield is higher than the estimatedvalue. The reactionis zero order with respectto benzeneand approxi- mately first order with respect to steam. In the reactionof alkylbenzenes-steam,ring breakdownand dealkylationoccur at the initial stage of reaction, and theformer occursmore easily than the latter at high conversionand at high temperature. The number and the position of the alkyl group on the benzene ring influencethe reaction rate and the selectivity. The reactionpath of dealkylationis proposed as follows: most effective catalyst for dealkylation with 1 Introduction steam, and they investigated reactivity and reac- The catalytic reaction of hydrocarbon with tion path on Ni/BeO at 450℃. steam is important for the production of hydrogen, In the present work, the reactions of benzene synthesis gas and town gas. The authors have or alkylbenzenes with steam were carried out previously reported the works on olefins-steam over a silica-supported nickel catalyst under and paraffins-steam reactions over a silica-sup- atmospheric pressure in a temperature range of ported nickel catalyst1)~3), and this study on 370~430℃, and the effects of reaction conditions, aromatics-steam reactions has been carried out rate of each reaction and the reaction path were as a consecutive one. -

Disproportionation and Transalkylation of Alkylbenzenes Over Zeolite Catalysts
Applied Catalysis A: General 181 (1999) 355±398 Disproportionation and transalkylation of alkylbenzenes over zeolite catalysts Tseng-Chang Tsaia, Shang-Bin Liub, Ikai Wangc,* aRe®ning and Manufacturing Research Center, Chinese Petroleum Corporation, Chiayi 600, Taiwan bInstitute of Atomic and Molecular Sciences, Academia Sinica, PO Box 23-166, Taipei 106, Taiwan cDepartment of Chemical Engineering, National Tsing-Hua University, Hsinchu 300, Taiwan Received 13 June 1998; received in revised form 3 October 1998; accepted 5 November 1998 Abstract Disproportionation and transalkylation are important processes for the interconversion of mono-, di-, and tri-alkylbenzenes. In this review, we discuss the recent advances in process technology with special focus on improvements of para-isomer selectivity and catalyst stability. Extensive patent search and discussion on technology development are presented. The key criteria for process development are identi®ed. The working principles of para-isomer selectivity improvements involve the reduction of diffusivity and the inactivation of external surface. In conjunction with the fundamental research, various practical modi®cation aspects particularly the pre-coking and the silica deposition techniques, are extensively reviewed. The impact of para-isomer selective technology on process economics and product recovery strategy is discussed. Furthermore, perspective trends in related research and development are provided. # 1999 Elsevier Science B.V. All rights reserved. Keywords: Disproportionation; Transalkylation; -

Trimethylbenzenes CAS Registry Numbers: 526-73-6 (1,2,3-TMB) 95-63-6 (1,2,4-TMB) 108-67-8 (1,3,5-TMB) 25551-13-7 (Mixed Isomers)
Development Support Document Final, September 4, 2015 Trimethylbenzenes CAS Registry Numbers: 526-73-6 (1,2,3-TMB) 95-63-6 (1,2,4-TMB) 108-67-8 (1,3,5-TMB) 25551-13-7 (Mixed Isomers) Prepared by Joseph T. Haney, Jr., M.S. Angela Curry, M.S. Toxicology Division Office of the Executive Director TEXAS COMMISSION ON ENVIRONMENTAL QUALITY Trimethylbenzenes Page i TABLE OF CONTENTS TABLE OF CONTENTS ............................................................................................................................................ I LIST OF TABLES ......................................................................................................................................................II ACRONYMS AND ABBREVIATIONS ................................................................................................................. III CHAPTER 1 SUMMARY TABLES .......................................................................................................................... 1 CHAPTER 2 MAJOR SOURCES AND USES ......................................................................................................... 4 CHAPTER 3 ACUTE EVALUATION ...................................................................................................................... 4 ACUTE 3.1 HEALTH-BASED ACUTE REV AND ESL ........................................................................................................ 4 3.1.1 Physical/Chemical Properties .................................................................................................................... -

Targeting Glycine Reuptake in Alcohol Seeking and Relapse
JPET Fast Forward. Published on January 24, 2018 as DOI: 10.1124/jpet.117.244822 This article has not been copyedited and formatted. The final version may differ from this version. TITLE PAGE Targeting Glycine Reuptake in Alcohol Seeking and Relapse Valentina Vengeliene, Martin Roßmanith, Tatiane T. Takahashi, Daniela Alberati, Berthold Behl, Anton Bespalov, Rainer Spanagel Downloaded from The primary laboratory of origin: Institute of Psychopharmacology, Central Institute of jpet.aspetjournals.org Mental Health, Faculty of Medicine Mannheim, Heidelberg University, Germany; at ASPET Journals on September 30, 2021 VV, MR, TTT, RS: Institute of Psychopharmacology, Central Institute of Mental Health, Faculty of Medicine Mannheim, Heidelberg University, Germany; DA: Roche Pharma Research and Early Development, Neuroscience, Ophthalmology and Rare Diseases, Roche Innovation Center Basel, CH-4070 Basel, Switzerland; BB, AB: Department of Neuroscience Research, AbbVie Deutschland GmbH & Co. KG, Ludwigshafen, Germany; AB: Department of Psychopharmacology, Pavlov Medical University, St Petersburg, Russia JPET #244822 JPET Fast Forward. Published on January 24, 2018 as DOI: 10.1124/jpet.117.244822 This article has not been copyedited and formatted. The final version may differ from this version. RUNNING TITLE GlyT1 in Alcohol Seeking and Relapse Corresponding author with complete address: Valentina Vengeliene, Institute of Psychopharmacology, Central Institute of Mental Health (CIMH), J5, 68159 Mannheim, Germany Email: [email protected], phone: +49-621-17036261; fax: +49-621- Downloaded from 17036255 jpet.aspetjournals.org The number of text pages: 33 Number of tables: 0 Number of figures: 6 Number of references: 44 at ASPET Journals on September 30, 2021 Number of words in the Abstract: 153 Number of words in the Introduction: 729 Number of words in the Discussion: 999 A recommended section assignment to guide the listing in the table of content: Drug Discovery and Translational Medicine 2 JPET #244822 JPET Fast Forward. -

Reactions of Benzene & Its Derivatives
Organic Lecture Series ReactionsReactions ofof BenzeneBenzene && ItsIts DerivativesDerivatives Chapter 22 1 Organic Lecture Series Reactions of Benzene The most characteristic reaction of aromatic compounds is substitution at a ring carbon: Halogenation: FeCl3 H + Cl2 Cl + HCl Chlorobenzene Nitration: H2 SO4 HNO+ HNO3 2 + H2 O Nitrobenzene 2 Organic Lecture Series Reactions of Benzene Sulfonation: H 2 SO4 HSO+ SO3 3 H Benzenesulfonic acid Alkylation: AlX3 H + RX R + HX An alkylbenzene Acylation: O O AlX H + RCX 3 CR + HX An acylbenzene 3 Organic Lecture Series Carbon-Carbon Bond Formations: R RCl AlCl3 Arenes Alkylbenzenes 4 Organic Lecture Series Electrophilic Aromatic Substitution • Electrophilic aromatic substitution: a reaction in which a hydrogen atom of an aromatic ring is replaced by an electrophile H E + + + E + H • In this section: – several common types of electrophiles – how each is generated – the mechanism by which each replaces hydrogen 5 Organic Lecture Series EAS: General Mechanism • A general mechanism slow, rate + determining H Step 1: H + E+ E El e ctro - Resonance-stabilized phile cation intermediate + H fast Step 2: E + H+ E • Key question: What is the electrophile and how is it generated? 6 Organic Lecture Series + + 7 Organic Lecture Series Chlorination Step 1: formation of a chloronium ion Cl Cl + + - - Cl Cl+ Fe Cl Cl Cl Fe Cl Cl Fe Cl4 Cl Cl Chlorine Ferric chloride A molecular complex An ion pair (a Lewis (a Lewis with a positive charge containing a base) acid) on ch lorine ch loronium ion Step 2: attack of -

Summary of Gas Cylinder and Permeation Tube Standard Reference Materials Issued by the National Bureau of Standards
A111D3 TTbS?? o z C/J NBS SPECIAL PUBLICATION 260-108 o ^EAU U.S. DEPARTMENT OF COMMERCE/National Bureau of Standards Standard Reference Materials: Summary of Gas Cylinder and Permeation Tube Standard Reference Materials Issued by the National Bureau of Standards QC 100 U57 R. Mavrodineanu and T. E. Gills 260-108 1987 m he National Bureau of Standards' was established by an act of Congress on March 3, 1901. The Bureau's overall goal i s t0 strengthen and advance the nation's science and technology and facilitate their effective application for public benefit. To this end, the Bureau conducts research to assure international competitiveness and leadership of U.S. industry, science arid technology. NBS work involves development and transfer of measurements, standards and related science and technology, in support of continually improving U.S. productivity, product quality and reliability, innovation and underlying science and engineering. The Bureau's technical work is performed by the National Measurement Laboratory, the National Engineering Laboratory, the Institute for Computer Sciences and Technology, and the Institute for Materials Science and Engineering. The National Measurement Laboratory Provides the national system of physical and chemical measurement; • Basic Standards 2 coordinates the system with measurement systems of other nations and • Radiation Research furnishes essential services leading to accurate and uniform physical and • Chemical Physics chemical measurement throughout the Nation's scientific community, • Analytical Chemistry industry, and commerce; provides advisory and research services to other Government agencies; conducts physical and chemical research; develops, produces, and distributes Standard Reference Materials; provides calibration services; and manages the National Standard Reference Data System. -

BENZENE AS a LARVICIDE for SCREW WORMS1 the Larval Stage
BENZENE AS A LARVICIDE FOR SCREW WORMS1 By D. C. PARMAN Assistant Entomologist, Investigations of Insects Affecting the Health of Animals, Bureau of Entomology, United States Department of Agriculture INTRODUCTION The larval stage of CocMiomyia macellaria Fab., generally known amonff stock raisers in the Southwest as the screw worm, causes con- siderable loss to the livestock industry, estimated as high as $5,000,000 in some years. It has been apparent that the larvicides used to kill the worms are either toxic to the animal or at least in most cases detrimental to the healing of the wounds. This toxicity was at first attributed to the screw worm, but as many cases were ob- served where the animal was practically consumed by the larvae and still lived until the loss of olood or injury to some vital organ brought de^th, it was surmised that the treatments with larvicides were the cause of many deaths. During the summer of 1916 syste- matic work was begun to find a more efficient larvicide than the phenols and chloroform which were generally used. At first an attempt was made to add something to these larvicides to counteract the toxic properties. As this was not successful it was deemed best to look for a chemical that might be used with more satis- factory results. Several chemical groups were studied for possible larvicides. EXPERIMENTAL PROCEDURE All available chemicals with possible larvicidal value were selected for laboratory tests to determine whether they would kill the larvae of the screw-worm fly. The first tests were made by pouring the chemical on a number of larvae in a tube, or dusting on just enough to cover the larvae.