Journal of Current and Advance Medical Research a Case
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
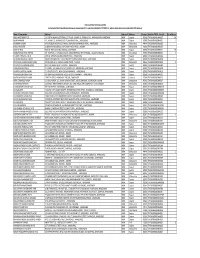
Statement of Unclaimed Dividend Amount Consecutively for 7 Years from Dividend of FY 2010-11, Whose Shares Are to Be Transferred to IEPF Account
THE ANUP ENGINEERING LIMITED Statement of Unclaimed Dividend amount consecutively for 7 years from Dividend of FY 2010-11, whose shares are to be transferred to IEPF Account Name of Shareholder Address-1 Address-2 Address-3 Pincode Folio No / DP ID - Client ID No. of Shares AEGIS INVESTMENTS LTD C/O.SHETH LALBHAI DALPATBHAI, 1ST FLOOR, 'AKSHAY' 53, SHRIMALI SOC., NAVRANGPURA, AHMEDABAD INDIA Gujarat 380009 THEA0000000000A00179 306 ANJANA MANAN 101, AKSHAY, 53, SHRIMALI SOCIETY, NAVRANGPURA,, , AHMEDABAD INDIA Gujarat 380009 THEA0000000000A00217 11 ARJANBHAI ALABHAI 664 KUBERDAS MODI'S OLD CHAWAL, SAIJPUR BOGHA NARODA ROAD, , AHMEDABAD INDIA Maharashtra 444444 THEA0000000000A00203 1 BAKULA R JHAVERI 10 SEKHSARIA BUILDINGS, 2ND FLOOR, 448 SVP ROAD, , MUMBAI INDIA Maharashtra 400004 THEA0000000000B00168 1 BANK OF INDIA BANK OF INDIA BUILDING, BHADRA, , AHMEDABAD INDIA Gujarat 380001 THEA0000000000B00118 783 BHARATKUMAR SHIVJI SHETHIA 7/1 'TILOTTMA', 1-C PANKAJ MULLICK, SARANI (FORMALY RITCHIE ROAD), , CALCUTTA KOLKATA INDIA West Bengal 700019 THEA0000000000B00138 33 C V MEHTA PRIVATE LIMITED BANK OF BARODA BUILDING, GANDHI ROAD, , AHMEDABAD INDIA Gujarat 380001 THEA0000000000C00068 1 DR ARUNA RAMANLAL JHAVERI RAJAN SHRI NIWAS SOC., SUKHIPURA NEW SHARDA MANDIR ROAD, , AHMEDABAD INDIA Gujarat 380007 THEA0000000000A00086 1 DR JIVANLAL HUKAMCHANDJI PAREKH C/O.DHANRAJ & CO., RESHAM BAZAR ITWARI, , NAGPUR INDIA Maharashtra 444444 THEA0000000000J00051 13 DR SUMATILAL VIRCHAND MODI NO.1 NEW ANJALI SOCIETY, VASANA, , AHMEDABAD INDIA Gujarat 380007 -
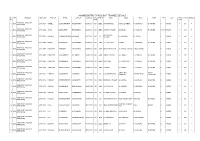
ANAND DISTRICT PASS out TRAINEE DETAILS Sr
ANAND DISTRICT PASS OUT TRAINEE DETAILS Sr. YRC TRADE_N SEAT_NO FIRST_N NAME LAST_N B_DATE PHY_ CASTE ADD1 ADD2 ADD3 ADD4 PIN ITI_N TRIA GTOTAL RESULT No. HAND L_NO ARMATURE & MOTOR 1 2010 216811001 GOHEL JAGDISHKUMAR MADHAVBHAI 01/06/1987 NO SEBC SUTHAR FALIU AT&PO:-DAHEMI TA:-BORSAD DI:-ANAND 0 VASAD 1 533 P REWINDING ARMATURE & MOTOR 2 2010 216811002 JADAV DILIPKUMAR BHANUBHAI 04/09/1988 NO SEBC GANPATI CHOWK AT:-ZILOD TA:-ANKLAV DI:-ANAND 388510 VASAD 1 514 P REWINDING ARMATURE & MOTOR OPP-BHARAT 3 2010 216811003 KALASVA RAVINDRAKUMAR KALUBHAI 07/11/1993 NO ST AT:-BORSAD DI:-ANAND 0 VASAD 1 543 P REWINDING SAWMILL ARMATURE & MOTOR 4 2010 216811004 MALEK IMRANKHAN ANAVARBHAI 07/06/1991 NO GEN AT:-VADI FALIA PAMOL TA:-BORSAD DI:-ANAND 0 VASAD 1 593 P REWINDING ARMATURE & MOTOR 5 2010 216811005 PADHIYAR AMBUBHAI CHIMANBHAI 01/06/1991 NO GEN BHAGVAN NAGAR AT:-NAPAD TALPAD TA&DI:-ANAND 0 VASAD 1 564 P REWINDING ARMATURE & MOTOR 6 2010 216811007 PADHIYAR JAGDISHBHAI NATUBHAI 23/05/1992 NO GEN AMBAV PARAMA AT:-AMBAV TA:-ANKLAV DI:-ANAND 0 VASAD 1 568 P REWINDING ARMATURE & MOTOR 7 2010 216811008 PADHIYAR MAHESHBHAI GOVINDBHAI 22/12/1990 NO SEBC MOTIPURA AT:-JOSHIKUVA TA:-ANKLAV DI:-ANAND 0 VASAD 1 549 P REWINDING ARMATURE & MOTOR 8 2010 216811010 PADHIYAR SANJAYKUMAR CHANDUBHAI 13/08/1993 NO GEN PARAMA AT:-AMBAV TA:-ANKLAV DI:-ANAND 0 VASAD 1 619 P REWINDING ARMATURE & MOTOR OPP-ALMAC TA&DI:- 9 2010 216811011 PARMAR ALPESHBHAI KANUBHAI 03/11/1990 NO SC A.23,GANHNAGAR CHHANI ROAD 0 VASAD 1 545 P REWINDING COMPANY VADODARA ARMATURE & MOTOR -
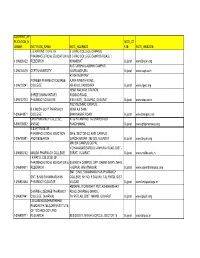
Current Ap Plication Umber N Institute Name Insti Address Insti St Ate Insti Website 1-396085422 L B Rao Institute of Pharmac
CURRENT_AP PLICATION_N INSTI_ST UMBER INSTITUTE_NAME INSTI_ADDRESS ATE INSTI_WEBSITE L B RAO INSTITUTE OF B D RAO COLLEGE CAMPUS, PHARMACEUTICAL EDUCATION & B D RAO COLLEGE CAMPUS ROAD, 1-396085422 RESEARCH KHAMBHAT Gujarat www.lbriper.org KASTURBHAI LALBHAI CAMPUS 1-396100415 CEPT UNIVERSITY NAVRANGPURA Gujarat www.cept.ac.in AT-SAYAJIPYRA PIONEER PHARMACY DEGREE AJWA-NIMETA ROAD 1-396103241 COLLEGE NR-N.H.8, VADODARA Gujarat www.ogect.org NEAR RAILWAY STATION, SHREE DHANVANTARY KUDSAD ROAD 1-396137213 PHARMACY COLLEGE KIM (EAST), TA-OLPAD, DI-SURAT Gujarat www.sdpc.co.in POLYTECHNIC CAMPUS, B.K.MODY GOVT.PHARMACY NEAR AJI DAM 1-396649871 COLLEGE BHAVNAGAR ROAD Gujarat www.bkmgpc.com GHB PHARMACY COLLEGE, AT & PO-ANIYAD,,, TA-SHAHERA,DI- 1-396708382 ANIYAD PANCHMAHAL Gujarat www.ghbpharmacy.org K.B.INTITUTE OF PHARMACEUTICAL EDUCTION GH/6, SECTOR-23, KADI CAMPUS, 1-396780981 AND RESEARCH GANDHINAGAR- 382 023, GUJARAT Gujarat www.kbiper.org MALIBA CAMPUS,GOPAL VIDYANAGAR,BARDOLI-MAHUVA ROAD, DIST - 1-396882142 MALIBA PHARMACY COLLEGE SURAT, GUJARAT Gujarat www.maliba.edu.in I.K.PATEL COLLEGE OF PHARMACEUTICAL EDUCATION & SAMARTH CAMPUS, OPP. SABAR DAIRY, NH-8, 1-396899501 RESEARCH HAJIPUR, HIMATNAGAR Gujarat www.samarthcampus.com SMT. B.N.B. SWAMINARAYAN PHARMACY SMT. B.N.B SWAMINARAYAN COLLEGE, NH NO. 8 SALVAV, TAL PARDI, DIST 1-396901664 PHARMACY COLLEGE VALSAD Gujarat www.bnbspcollege.in AMRAPALI TOWNSHIP, PETLAD-KHAMBHAT DHARMAJ DEGREE PHARMACY ROAD, DHARMAJ-388430 1-396907441 COLLEGE, DHARMAJ TA: PETLAD, DIST: ANAND, GUJARAT Gujarat www.ipcprc.org LEELABEN DASHRATHBHAI RAMDAS PATEL(LDRP)INSTITUTE OF TECHNOLOGY AND 1-396908711 RESEARCH BESIDES ITI, NR KH 5 CIRCLE, SECTOR 15 Gujarat www.ldrp.ac.in KRISHNA KAMPUS, BECHRAJI-SHANKHALPUR SHREE KRISHNA INSTITUTE OF ROAD, TALUKA-BECHRAJI, DIST-MEHSANA, 1-396910173 PHARMACY GUJARAT Gujarat www.skip.org.in MEHSANA - VISNAGAR HIGHWAY, AT & PO. -
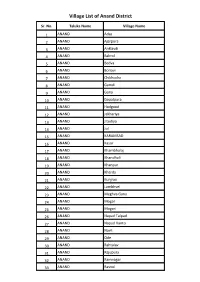
Village List of Anand District
Village List of Anand District Sr. No. Taluka Name Village Name 1 ANAND Adas 2 ANAND Ajarpura 3 ANAND Anklavdi 4 ANAND Bakrol 5 ANAND Bedva 6 ANAND Boriavi 7 ANAND Chikhodra 8 ANAND Gamdi 9 ANAND Gana 10 ANAND Gopalpura 11 ANAND Hadgood 12 ANAND Jakhariya 13 ANAND Jitodiya 14 ANAND Jol 15 ANAND KARAMSAD 16 ANAND Kasor 17 ANAND Khambholaj 18 ANAND Khandhali 19 ANAND Khanpur 20 ANAND Kherda 21 ANAND Kunjrao 22 ANAND Lambhvel 23 ANAND Meghva Gana 24 ANAND Mogar 25 ANAND Mogari 26 ANAND Napad Talpad 27 ANAND Napad Vanto 28 ANAND Navli 29 ANAND Ode 30 ANAND Rahtalav 31 ANAND Rajupura 32 ANAND Ramnagar 33 ANAND Rasnol 34 ANAND Samarkha 35 ANAND Sandesar 36 ANAND Sarsa 37 ANAND Sundan 38 ANAND Tarnol 39 ANAND Vadod 40 ANAND Vaghasi 41 ANAND Vaherakhadi 42 ANAND Valasan 43 ANAND Vans Khiliya 44 ANAND Vasad Village List of Petlad Taluka Sr. No. Taluka Name Village Name 1 PETLAD Agas 2 PETLAD Amod 3 PETLAD Ardi 4 PETLAD Ashi 5 PETLAD Bamroli 6 PETLAD Bandhani 7 PETLAD Bhalel 8 PETLAD Bhatiel 9 PETLAD Bhavanipura 10 PETLAD Bhurakui 11 PETLAD Boriya 12 PETLAD Changa 13 PETLAD Dantali 14 PETLAD Danteli 15 PETLAD Davalpura 16 PETLAD Demol 17 PETLAD Dhairyapura 18 PETLAD Dharmaj 19 PETLAD Fangani 20 PETLAD Ghunteli 21 PETLAD Isarama 22 PETLAD Jesarva 23 PETLAD Jogan 24 PETLAD Kaniya 25 PETLAD Khadana 26 PETLAD Lakkadpura 27 PETLAD Mahelav 28 PETLAD Manej 29 PETLAD Manpura 30 PETLAD Morad 31 PETLAD Nar 32 PETLAD Padgol 33 PETLAD Palaj 34 PETLAD Pandoli 35 PETLAD Petlad 36 PETLAD Porda 37 PETLAD Ramodadi 38 PETLAD Rangaipura 39 PETLAD Ravipura 40 PETLAD Ravli 41 PETLAD Rupiyapura 42 PETLAD Sanjaya 43 PETLAD Sansej 44 PETLAD Shahpur 45 PETLAD Shekhadi 46 PETLAD Sihol 47 PETLAD Silvai 48 PETLAD Simarada 49 PETLAD Sunav 50 PETLAD Sundara 51 PETLAD Sundarana 52 PETLAD Vadadala 53 PETLAD Vatav 54 PETLAD Virol(Simarada) 55 PETLAD Vishnoli 56 PETLAD Vishrampura Village List of Borsad Taluka Sr. -
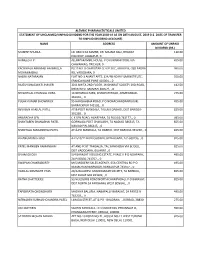
Name Address Amount of Unpaid Dividend (Rs.) Mukesh Shukla Lic Cbo‐3 Ka Samne, Dr
ALEMBIC PHARMACEUTICALS LIMITED STATEMENT OF UNCLAIMED/UNPAID DIVIDEND FOR THE YEAR 2018‐19 AS ON 28TH AUGUST, 2019 (I.E. DATE OF TRANSFER TO UNPAID DIVIDEND ACCOUNT) NAME ADDRESS AMOUNT OF UNPAID DIVIDEND (RS.) MUKESH SHUKLA LIC CBO‐3 KA SAMNE, DR. MAJAM GALI, BHAGAT 110.00 COLONEY, JABALPUR, 0 HAMEED A P . ALUMPARAMBIL HOUSE, P O KURANHIYOOR, VIA 495.00 CHAVAKKAD, TRICHUR, 0 KACHWALA ABBASALI HAJIMULLA PLOT NO. 8 CHAROTAR CO OP SOC, GROUP B, OLD PADRA 990.00 MOHMMADALI RD, VADODARA, 0 NALINI NATARAJAN FLAT NO‐1 ANANT APTS, 124/4B NEAR FILM INSTITUTE, 550.00 ERANDAWANE PUNE 410004, , 0 RAJESH BHAGWATI JHAVERI 30 B AMITA 2ND FLOOR, JAYBHARAT SOCIETY 3RD ROAD, 412.50 KHAR WEST MUMBAI 400521, , 0 SEVANTILAL CHUNILAL VORA 14 NIHARIKA PARK, KHANPUR ROAD, AHMEDABAD‐ 275.00 381001, , 0 PULAK KUMAR BHOWMICK 95 HARISHABHA ROAD, P O NONACHANDANPUKUR, 495.00 BARRACKPUR 743102, , 0 REVABEN HARILAL PATEL AT & POST MANDALA, TALUKA DABHOI, DIST BARODA‐ 825.00 391230, , 0 ANURADHA SEN C K SEN ROAD, AGARPARA, 24 PGS (N) 743177, , 0 495.00 SHANTABEN SHANABHAI PATEL GORWAGA POST CHAKLASHI, TA NADIAD 386315, TA 825.00 NADIAD PIN‐386315, , 0 SHANTILAL MAGANBHAI PATEL AT & PO MANDALA, TA DABHOI, DIST BARODA‐391230, , 0 825.00 B HANUMANTH RAO 4‐2‐510/11 BADI CHOWDI, HYDERABAD, A P‐500195, , 0 825.00 PATEL MANIBEN RAMANBHAI AT AND POST TANDALJA, TAL.SANKHEDA VIA BODELI, 825.00 DIST VADODARA, GUJARAT., 0 SIVAM GHOSH 5/4 BARASAT HOUSING ESTATE, PHASE‐II P O NOAPARA, 495.00 24‐PAGS(N) 743707, , 0 SWAPAN CHAKRABORTY M/S MODERN SALES AGENCY, 65A CENTRAL RD P O 495.00 -

Directory Establishment
DIRECTORY ESTABLISHMENT SECTOR :RURAL STATE : GUJARAT DISTRICT : Ahmadabad Year of start of Employment Sl No Name of Establishment Address / Telephone / Fax / E-mail Operation Class (1) (2) (3) (4) (5) NIC 2004 : 0121-Farming of cattle, sheep, goats, horses, asses, mules and hinnies; dairy farming [includes stud farming and the provision of feed lot services for such animals] 1 VIJAYFARM CHELDA , PIN CODE: 382145, STD CODE: NA , TEL NO: 0395646, FAX NO: NA, E-MAIL : N.A. NA 10 - 50 NIC 2004 : 1020-Mining and agglomeration of lignite 2 SOMDAS HARGIVANDAS PRAJAPATI KOLAT VILLAGE DIST.AHMEDABAD PIN CODE: NA , STD CODE: NA , TEL NO: NA , FAX NO: NA, 1990 10 - 50 E-MAIL : N.A. 3 NABIBHAI PIRBHAI MOMIN KOLAT VILLAGE DIST AHMEDABAD PIN CODE: NA , STD CODE: NA , TEL NO: NA , FAX NO: NA, 1992 10 - 50 E-MAIL : N.A. 4 NANDUBHAI PATEL HEBATPUR TA DASKROI DIST AHMEDABAD , PIN CODE: NA , STD CODE: NA , TEL NO: NA , 2005 10 - 50 FAX NO: NA, E-MAIL : N.A. 5 BODABHAI NO INTONO BHATHTHO HEBATPUR TA DASKROI DIST AHMEDABAD , PIN CODE: NA , STD CODE: NA , TEL NO: NA , 2005 10 - 50 FAX NO: NA, E-MAIL : N.A. 6 NARESHBHAI PRAJAPATI KATHAWADA VILLAGE DIST AHMEDABAD PIN CODE: 382430, STD CODE: NA , TEL NO: NA , 2005 10 - 50 FAX NO: NA, E-MAIL : N.A. 7 SANDIPBHAI PRAJAPATI KTHAWADA VILLAGE DIST AHMEDABAD PIN CODE: 382430, STD CODE: NA , TEL NO: NA , FAX 2005 10 - 50 NO: NA, E-MAIL : N.A. 8 JAYSHBHAI PRAJAPATI KATHAWADA VILLAGE DIST AHMEDABAD PIN CODE: NA , STD CODE: NA , TEL NO: NA , FAX 2005 10 - 50 NO: NA, E-MAIL : N.A. -

David Hardiman . Submitted for the Degree of Doctor
'ý cv (Ti 1 ýýýý +ý e " :` -10ý e ýý tS; " iii, ' ýýI Vý u, I, ' 'ý l `r .ý 3 ." ?ý j Peasant Agitations in Kheda Disttiýt, Gujarat, 1917 -1934. David Hardiman . Submitted for the degree of Doctor of Philosophy at the University of Sussex, September 1975. Copy number: i2 AHMEDABAD Kap-advani KathIaI " Mehmedabad "" Thasra Mahuda " Kheda Dakor Matar " " Nadiad Umreth "cis "Vadta Anand " So*itrci"/ Kdaramsa " Pet Dharmj Borsad " Virs p dran BARODA " -CAMBAY Mahi Kheda District during the period of British -Rule. Taluka Headquarter Other Places "Anand Town Vaso of Importance Cambay State Parts of Baroda State CO;I" E YYTS Page No . List of Maps i List of Abbreviations used in footnotes ii Introduction iii-ix CHAPTER Oi;E: THL G'U^rRJPHY Ji''D PEOPLE OF NIN2TEENTH CLNTURY YJ D;ý I CHAPTER Tv;O: THE STRUCTUREOF LOCi:L DO}.'INAI C 23 1. The Traditional Village Structure 23 2. The Rise cf some Leading :tianbis within the Traditional Bureaucratic Syste: 29 -i 3. The Impact of British Rule on the Traditional Structure 34 4.. Standing within the Caste 40 c::I1APTFR THREE :I 'GOLDEN AGE' FOR THE K0 BI S 44 1. The Aristocratic Kanbis 44 2. The Superior Kandis 4-9 3. The Lesser Kanbis 55 4. From Kanbi to Patidar 61 5. The Tradition of the bhakti sect 63 6. Peasant Impressions of the British 68 CHAPTER FOUR: THE YEARS OF DISASTER 73 1. The Famine 73 2. The Growth of Discontent 80 C1L&PIER FIVE: THE DES :LO?, ', TT OF A NtiTIO?«. -

Rajesh Patel (Dharmaj ) C. V . 2017
Rajesh Patel (Dharmaj ) C. V . 2017 Personal Details: Name : Rajesh Father’s Name : Rameshbhai D. Patel Mother’s Name : Shardaben Patel Native : Dharmaj Dist: Anand Date of Birth : 11th August 1959 Age: 57 Yrs. Place of Birth : Sojitra Dist: Anand (Maternal Town) Contact Details: Address : 18, Shivalay Bunglows, Near Ramdev Mandir, At: Karamsad – 388 325 Contact No. : Cell No. 9426 500 757 & 9408 150 500 (Res.) 02692 – 22 22 23 Email ID : [email protected] / [email protected] : facebook/rajesh.pateldharmaj / twitter@rajudharmaj Introduction: : Fondly called ‘Raju Dharmaj’ means a man of commitment, a man of Society, a man of Goodwill and a man who lives for motherland. Educational Qualification: : Diploma Electrical Engineer (1981) Diploma Mechanical Engineer (1984) Diploma in Computer Applications. (1992) Profession: : Worked at Eimco Elecon India Ltd. as a Junior Engineer. : Engaged in Trading of Mill Jin Stores Machineries. : Engaged in Manufacturing Unit of own. : looking after family owned Agriculture Land. Nobel Cause: : Rajesh Patel rendering services as an Hon. Advisor to Shri Jalaram Jan Seva Trust, Dharmaj. Dharmaj Day: : An initiative taken by Rajesh Patel in the year 2007. It has completed 11 successful Years in 2017. Publications: : Authored 7 books and a coffee table book about Dharmaj. Name the few are, : Title –‘Jivan Path Na Pagathiya ‘ Gujarati (20000 Copies) – Best Seller Gujarati Book Title – ‘Tantravali’ published by Navbharat Prakashan Mandir, Amadavad. 1 of 3 : Title – ‘Dharmaj – Ek Udaharniya Gam‘ Gujarati (4000 copies) and English addition ‘Dharmaj – A Village to Emulate’ (1000 copies) published by Dharmaj Day Celebration Committee. : Title – ‘Bhajapa Sangathan – Mara Anubhavo’ in Gujarati ( 2000 Copies ) published by Sharda Foundation. -

A Local Community
UvA-DARE (Digital Academic Repository) Mobility and the region: A multi-scalar ethnography of the Vohra Gujarati community, in India and abroad Verstappen, S.B. Publication date 2016 Document Version Final published version Link to publication Citation for published version (APA): Verstappen, S. B. (2016). Mobility and the region: A multi-scalar ethnography of the Vohra Gujarati community, in India and abroad. General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:11 Oct 2021 2 PART PART A local community CHAPTER 3 | A ‘MUSLIM AREA’ IN A REGIONAL TOWN | 67 Town Planning Map of Anand, courtesy Anand municipality (2012). Vallabh Vidyanagar is on the left, Anand in the middle, Gamdi on the right. 68 CHAPTER 3 2 A ‘Muslim area’ PART in a regional town Today, if one approaches Anand from the main road that connects it to the cities of Ahmedabad and Vadodara (Baroda), a high bridge provides a view of a sprawling Town Planning Map of Anand, courtesy Anand municipality (2012). -

All College List GIA-SF
SARDAR PATEL UNIVERSITY AFFILIATED COLLEGES 1 Principal 2 Principal Anand Mercantile College of Science, AIMS College of Management & Technology Management & Computer Technology, AIMS Education Campus, Bhalej Road, Vadtal Road, Anand - 388 001. (SFI) Bakrol – 388 001. (SFI) 3 Principal 4 Principal Akhilesh Sureshbhai Patel Arts College, Anand Arts College, At. & Po. Boriavi – 387 310 Sh. Ramkrishna S eva Mandal , Ta. & Dist. Anand (SFI) Nr. Grid, Anand - 388 001 (GIA/SFI) 5 Principal 6 Principal Anand College of Education, Anand Commerce College, Sh. Ramkrishna Seva Mandal, Sh. Ramkrishna Seva M andal, Nr. Town Hall, Anand - 388 001. (SFI) Nr. Town Hall, Anand - 388 001. (GIA/SFI) 7 Principal 8 Principal Anand Education College, Anand Homoeopathic Medical College & Sh. Ramkrishna Seva Mandal, Research Institute, Sh. Ramkrishna Seva Nr. Town Hall, Mandal, Nr. Sardar Baug, Anand - 388 001. (GIA) Anand - 388 001 (GIA) 9 Principal 10 Principal Anand Institute of P.G. Studies In Arts, Anand Institute of Business Studies, Sh. Ramkrishna Seva Mandal, Sh. Ramkrishna Seva Mandal, Nr. Grid, Anand - 388 001 (SFI) Nr. Town Hall, Anand - 388 001. (SFI) 11 Principal 12 Principal Anand Institute of Social Work, Anand Law College, Sh. Ramkrishna Seva Mandal, Sh. Ramkrishna Seva Mandal, Nr. Town Hall, Nr. Town Hall , Anand - 388 001. (SFI) Anand - 388 001. (GIA/SFI) 13 Principal 14 Principal Ashok & Rita Patel Institute of Integrated Study Bhikhabhai Jivabhai Vanijya Mahavidyalaya &Research In Biotechnology And Allied Sciences (BJVM), (ARIBAS), Opp. Vitthal Udyognagar-GIDC, Vallabh Vidyanagar - 388 120. (GIA/SFI) Behind Phase-IV GIDC, New Vallabh Vidyanagar - 388 121. (SFI) 15 Principal 16 Principal B.N. -

Police Station
COURTWISE POLICE STATION Name of Establishment: Civil Court, Anand Sr.No Court Designation Police Station Anand Town Police Station 1 Chief Judicial Magistrate Vidhyanagar Police Station Anand Mahila Police Station Anand Rural Police Station 2 Additional Civil Judge & J.M.F.C Railway Protection Force, Anand [NC Cases] [RPF] M.G.V.C.L Police Station Anand Railway Police Station [GRP] 3 2nd Additional Civil Judge & J.M.F.C Khambholaj Police Station Vasad Police Station Name of Establishment: Civil Court, Borsad Sr.No Court Designation Police Station Principal Senior Civil Judge & 1 Borsad Rural Additional Chief Judicial Magistrate 2 Additional Sr. Civil Judge & A.C.J.M Borsad Town 3 Additional Civil Judge & J.M.F.C Bhadran, Ras 4 2nd Additional Civil Judge & J.M.F.C Virsad, O.P. Kathana Name of Establishment: Civil Court, Khambhat Sr.No Court Designation Police Station Principal Senior Civil Judge & 1 Khambhat Town Additional Chief Judicial Magistrate Khambhat Rural,O.P. Piploi, 2 Additional Senior Civil Judge & A.C.J.M. Dhuvaran, Kalamsar, Golana, Pandar Name of Establishment: Civil Court, Petlad Sr.No Court Designation Police Station Principal Senior Civil Judge & 1 Petlad Town,Petlad Rural Additional Chief Judicial Magistrate Mahelav, O.P. 2 Additional Civil Judge & J.M.F.C Dharmaj, Nar Name of Establishment: Civil Court, Umreth Sr.No Court Designation Police Station 1 Principal Civil Judge & J.M.F.C Umreth 2 Additional Civil Judge & J.M.F.C Khambholaj, Bhalej Name of Establishment: Civil Court, Sojitra Sr.No Court Designation Police Station 1 Principal Civil Judge & J.M.F.C Sojitra Police Station Name of Establishment: Civil Court, Tarapur Sr.No Court Designation Police Station 1 Principal Civil Judge & J.M.F.C Tarapur Police Station Name of Establishment: Civil Court, Anklav Sr.No Court Designation Police Station 1 Principal Civil Judge & J.M.F.C Anklav Police Station. -

Paid Pending Applications As on Aug'19
PAID PENDING APPLICATIONS AS ON AUG'19 Circle: Anand Tentative Work Involved Sr Date Of Reg FQ Issue Date FQ Paid Date Month Of Scheme Sub Div Division Village Taluka District Name Load cat Remark No Work HT LT T/C Complition DD MM YYYY KM KM Cap DD MM YYYY DD MM YYYY V.V.NAGA 1 SPA Anand City Bakrol Anand Anand Malek Murtujahusen ayubmiya 10 8 10 2018 C 0.19 16 to 25 22 7 2019 23 7 2019 Sep-19 R V.V.NAGA 2 SPA Anand City Bakrol Anand Anand Patel Kanubhai Jashbhai 10 19 11 2018 B 0.025 0 22 7 2019 29 7 2019 Sep-19 R V.V.NAGA 3 SPA Anand City Bakrol Anand Anand Khureshi Asarafmiya Hasumiya 10 29 11 2018 C 0.17 10 to 16 22 7 2019 1 8 2019 Sep-19 R V.V.NAGA ANAND 4 SPA BAKROL Anand Anand PATEL JYOTIBEN NILESHBHAI 10 18 12 2018 D 0.08 10 KVA 22 7 2019 24 7 2019 Oct-19 R CITY 5 SPA Anand-S ANAND Khanpur Anand Anand Bhagvansinh Vakhatsinh Chakabhai Chauhan 10 18 5 2018 D 0.17 10 KVA 17 5 2019 11 6 2019 Sep-19 6 SPA Anand-S Anand Chikhodra Anand Anand Vasantbhai Ambalal Patel 15 2 7 2018 C 25 to 63 26 8 2019 27 8 2019 Sep-19 7 SPA Umreth-R Anand Chunel Mahudha Kheda Ravjibhai Bhikhabhai Prajapati 10 3 7 2018 D 0.63 10 KVA 25 6 2019 27 6 2019 Nov-19 Way problem 8 SPA Umreth-R Anand Parvata Umreth Anand Kesharbhai Chunabhai Rathod 7.5 9 7 2018 C 0.05 16 to 25 25 6 2019 11 7 2019 Sep-19 Standing paddy 9 SPA Umreth-R Anand Chunel Mahudha Kheda Pravinbhai Bhikhabhai Patel 7.5 19 7 2018 C 0.3 10 to 16 25 6 2019 2 7 2019 Nov-19 crop.photographs taken.