Tropical Dermatology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 3 Bacterial and Viral Infections
GBB03 10/4/06 12:20 PM Page 19 Chapter 3 Bacterial and viral infections A mighty creature is the germ gain entry into the skin via minor abrasions, or fis- Though smaller than the pachyderm sures between the toes associated with tinea pedis, His customary dwelling place and leg ulcers provide a portal of entry in many Is deep within the human race cases. A frequent predisposing factor is oedema of His childish pride he often pleases the legs, and cellulitis is a common condition in By giving people strange diseases elderly people, who often suffer from leg oedema Do you, my poppet, feel infirm? of cardiac, venous or lymphatic origin. You probably contain a germ The affected area becomes red, hot and swollen (Ogden Nash, The Germ) (Fig. 3.1), and blister formation and areas of skin necrosis may occur. The patient is pyrexial and feels unwell. Rigors may occur and, in elderly Bacterial infections people, a toxic confusional state. In presumed streptococcal cellulitis, penicillin is Streptococcal infection the treatment of choice, initially given as ben- zylpenicillin intravenously. If the leg is affected, Cellulitis bed rest is an important aspect of treatment. Where Cellulitis is a bacterial infection of subcutaneous there is extensive tissue necrosis, surgical debride- tissues that, in immunologically normal individu- ment may be necessary. als, is usually caused by Streptococcus pyogenes. A particularly severe, deep form of cellulitis, in- ‘Erysipelas’ is a term applied to superficial volving fascia and muscles, is known as ‘necrotiz- streptococcal cellulitis that has a well-demarcated ing fasciitis’. This disorder achieved notoriety a few edge. -

Current Microbiological, Clinical and Therapeutic Aspects of Impetigo Lior Zusmanovich, Lior Charach and Gideon Charach*
ISSN: 2378-3656 Zusmanovich et al. Clin Med Rev Case Rep 2018, 5:205 DOI: 10.23937/2378-3656/1410205 Volume 5 | Issue 3 Clinical Medical Reviews Open Access and Case Reports CASE REPORT Current Microbiological, Clinical and Therapeutic Aspects of Impetigo Lior Zusmanovich, Lior Charach and Gideon Charach* Department of Internal Medicine “C”, Affiliated to Tel Aviv University, Israel *Corresponding author: Gideon Charach, Department of Internal Medicine “C”, Tel Aviv Sourasky Check for Medical Center, Sackler Medical School, Affiliated to Tel Aviv University, 6 Weizman Street, Tel Aviv updates 6423906, Israel, Tel: +972-3-6973766, Fax: +972-3-6973929, E-mail: [email protected] nonpurulent and purulent cellulitis, and treatment is Abstract based on extent of infection and risk factors. Abscesses Impetigo is a highly contagious infection of the epidermis, involve the dermis and deeper skin tissues as a result of seen especially among children, and transmitted through direct contact. Two bacteria are associated with impetigo: pus formation. S. aureus and GAS. Over 140 million people are suffering Impetigo is observed most frequently among chil- from impetigo at each time point, over 100 million are chil- dren. Two forms of impetigo exist, namely impetigo conta- dren 2-5 years of age and is transmitted through direct giosa, known as the non-bullous form and the second one contact [1]. Risk factors for impetigo include poor hy- being bullous impetigo which presents with large and fragile giene, low economic status, crowding and underlying bullae. Treatment options for impetigo include systemic an- scabies [2,3]. Important consideration is carriage of tibiotics, topical antibiotics as well as topical disinfectants. -

Kellie ID Emergencies.Pptx
4/24/11 ID Alert! recognizing rapidly fatal infections Susan M. Kellie, MD, MPH Professor of Medicine Division of Infectious Diseases, UNMSOM Hospital Epidemiologist UNMHSC and NMVAHCS Fever and…. Rash and altered mental status Rash Muscle pain Lymphadenopathy Hypotension Shortness of breath Recent travel Abdominal pain and diarrhea Case 1. The cross-country trucker A 30 year-old trucker driving from Oklahoma to California is hospitalized in Deming with fever and headache He is treated with broad-spectrum antibiotics, but deteriorates with obtundation, low platelet count, and a centrifugal petechial rash and is transferred to UNMH 1 4/24/11 What is your diagnosis? What is the differential diagnosis of fever and headache with petechial rash? (in the US) Tickborne rickettsioses ◦ RMSF Bacteria ◦ Neisseria meningitidis Key diagnosis in this case: “doxycycline deficiency” Key vector-borne rickettsioses treated with doxycycline: RMSF-case-fatality 5-10% ◦ Fever, nausea, vomiting, myalgia, anorexia and headache ◦ Maculopapular rash progresses to petechial after 2-4 days of fever ◦ Occasionally without rash Human granulocytotropic anaplasmosis (HGA): case-fatality<1% Human monocytotropic ehrlichiosis (HME): case fatality 2-3% 2 4/24/11 Lab clues in rickettsioses The total white blood cell (WBC) count is typicallynormal in patients with RMSF, but increased numbers of immature bands are generally observed. Thrombocytopenia, mild elevations in hepatic transaminases, and hyponatremia might be observed with RMSF whereas leukopenia -

Pediatric Invasive Gastrointestinal Fungal Infections: Causative Agents and Diagnostic Modalities
Microbiology Research Journal International 19(2): 1-11, 2017; Article no.MRJI.32231 Previously known as British Microbiology Research Journal ISSN: 2231-0886, NLM ID: 101608140 SCIENCEDOMAIN international www.sciencedomain.org Pediatric Invasive Gastrointestinal Fungal Infections: Causative Agents and Diagnostic Modalities Mortada H. F. El-Shabrawi 1, Lamiaa A. Madkour 2* , Naglaa Kamal 1 and Kerstin Voigt 3 1Department of Pediatrics, Faculty of Medicine, Cairo University, Egypt. 2Department of Microbiology and Immunology, Faculty of Medicine, Cairo University, Egypt. 3Department of Microbiology and Molecular Biology, University of Jena, Germany. Authors’ contributions This work was carried out in collaboration between all authors. Author MHFES specified the topic of the research. Author LAM designed the study, managed the literature research and wrote the first draft of the manuscript. Authors NK and KV wrote the subsequent drafts. Author MHFES revised the manuscript. All authors read and approved the final manuscript. Article Information DOI: 10.9734/MRJI/2017/32231 Editor(s): (1) Raúl Rodríguez-Herrera, Autonomous University of Coahuila, México. Reviewers: (1) Hasibe Vural, Necmettin Erbakan Üniversity, Turkey. (2) Berdicevsky Israela, Technion Faculty of Medicine, Haifa, Israel. (3) Vassiliki Pitiriga, University of Athens, Greece. Complete Peer review History: http://www.sciencedomain.org/review-history/18327 Received 16 th February 2017 th Review Article Accepted 18 March 2017 Published 24 th March 2017 ABSTRACT Invasive gastrointestinal fungal infections are posing a serious threat to the ever-expanding population of immunocompromised children, as well as some healthy children at risk. In this narrative review, we collate and explore the etiologies and diagnostic modalities of these overlooked infections. -

Granuloma De Las Piscinas Carcinoma Basocelular Localmente Avanzado
Penfigoide gestacional pustuloso Granuloma facial de localización atípica Programa de Educación Médica Continua Variantes de Archivos Argentinos de Dermatología clínicas inusuales de Año 2019, volumen 5, número 4 sarcoidosis Octubre, Noviembre y Diciembre 2019. cutánea Precio: $200 Carcinoma basocelular localmente avanzado tratado con Vismodegib Granuloma de las piscinas www.archivosdermato.org.ar [email protected] Educando Nos Programa de Educación Médica Continua de Archivos Argentinos de Dermatología Año 2019, volumen 5, número 4 Sumario Octubre, Noviembre y Diciembre 2019 Editorial Acrodermatitis enteropática 3 Glorio Roberto 36 Ruiz Díaz María, Kuen Bernardita, Caggia Antonella, Díaz Ysabel, Lozinsky Liliana 4 Reglamento de publicaciones Jornadas de Educación 40 Médica Continua Granuloma facial de 6 localización atípica Carcinoma basocelular Digilio Marianela, Manzano Roxana, Bendjuia Gabriela, 42 localmente avanzado tratado Schroh Roberto, Feinsilber Daniel con Vismodegib Van Caester Leandro Rodolfo, Alfaro María Florencia, Consigli Javier, De La Colina Marcelo, Manrique Valeria, Variantes clínicas inusuales de Pereyra Susana Beatriz 10 sarcoidosis cutánea Espósito Daniela, Márquez, Juan Manuel, López Di Noto Ada Laura, Marcucci Carolina, Sánchez Graciela, Merola Gladys Seguimiento en pacientes 46 sensibilizados a Queratosis folicular invertida de metilisotiazolinona 18 localización infrecuente Russo Juan Pedro, Palazzolo Juan Francisco, Adorni Romina, Consigli Carlos Landau Débora, Caruso Territoriale Antonella, Valente -

NEWSLETTER 2017•Issue 2
NEWSLETTER 2017•Issue 2 page 2 Deep dermatophytosis page 4 Deep dermatophytosis: A case report page 5 Fereydounia khargensis: A new and uncommon opportunistic yeast from Malaysia page 6 Itraconazole: A quick guide for clinicians Visit us at AFWGonline.com and sign up for updates Editors’ welcome Dr Mitzi M Chua Dr Ariya Chindamporn Adult Infectious Disease Specialist Associate Professor Associate Professor Department of Microbiology Department of Microbiology & Parasitology Faculty of Medicine Cebu Institute of Medicine Chulalongkorn University Cebu City, Philippines Bangkok, Thailand This year we celebrate the 8th year of AFWG: 8 years of pursuing excellence in medical mycology throughout the region; 8 years of sharing expertise and encouraging like-minded professionals to join us in our mission. We are happy to once again share some educational articles from our experts and keep you updated on our activities through this issue. Deep dermatophytosis may be a rare skin infection, but late diagnosis or ineffective treatment may lead to mortality in some cases. This issue of the AFWG newsletter focuses on this fungal infection that usually occurs in immunosuppressed individuals. Dr Pei-Lun Sun takes us through the basics of deep dermatophytosis, presenting data from published studies, and emphasizes the importance of treating superficial tinea infections before starting immunosuppressive treatment. Dr Ruojun Wang and Professor Ruoyu Li share a case of deep dermatophytosis caused by Trichophyton rubrum. In this issue, we also feature a new fungus, Fereydounia khargensis, first discovered in 2014. Ms Ratna Mohd Tap and Dr Fairuz Amran present 2 cases of F. khargensis and show how PCR sequencing is crucial to correct identification of this uncommon yeast. -

What Is Cosplay?
NO, REALLY: WHAT IS COSPLAY? Natasha Nesic A thesis submitted in partial fulfillment of the requirements for a B. A. in Anthropology with Honors. Mount Holyoke College May 2013 ACKNOWLEDGEMENTS “In anthropology, you can study anything.” This is what happens when you tell that to an impressionable undergrad. “No, Really: What is Cosplay?” would not have been possible without the individuals of the cosplay community, who gave their time, hotel room space, and unforgettable voices to this project: Tina Lam, Mario Bueno, Rob Simmons, Margaret Huey, Chris Torrey, Chris Troy, Calico Singer, Maxiom Pie, Cassi Mayersohn, Renee Gloger, Tiffany Chang, as well as the countless other cosplayers at AnimeNEXT, Anime Expo, and Otakon during the summer of 2012. A heap of gratitude also goes to Amanda Gonzalez, William Gonzalez, Kimberly Lee, Patrick Belardo, Elizabeth Newswanger, and Clara Bertagnolli, for their enthusiasm for this project—as well as their gasoline. And to my parents, Beth Gersh-Nesic and Dusan Nesic, who probably didn’t envision this eight years ago, letting me trundle off to my first animé convention in a homemade ninja getup and a face full of Watercolor marker. Many thanks as well to the Mount Holyoke College Anthropology Department, and the Office of Academic Deans for their financial support. Finally, to my advisor and mentor, Professor Andrew Lass: Mnogo hvala za sve. 1 Table of Contents Introduction.......................................................................................................................................3 -

Pulmonary Basidiobolomycosis: an Unusual Presentation in a Cancer Patient: a Case Report and Mini Review of Cases in India
Int.J.Curr.Microbiol.App.Sci (2015) 4(5): 798-805 ISSN: 2319-7706 Volume 4 Number 5 (2015) pp. 798-805 http://www.ijcmas.com Case Study Pulmonary Basidiobolomycosis: An unusual presentation in a cancer patient: A case report and mini review of cases in India Deba Dulal Biswal1, Manisa Sahu2* and Pallavi Bhaleker3 1Department of Medical Oncology, S L Raheja Hospital (A Fortis Associate) Mahim (W), Mumbai-400016, India 2Department of Microbiology, S L Raheja Hospital (A Fortis Associate) Mahim(W), Mumbai-400016, India *Corresponding author A B S T R A C T K e y w o r d s Basidiobolomycosis is an uncommon disease caused by Basidiobolus Basidiobolo- ranarum. The clinical presentation varies from localized subcutaneous mycosis, infection to widespread dissemination involving different viscera s, notably Basidio-bolus the gastrointestinal tract. Pulmonary involvement is rarer; we report a case ranarum, of pulmonary Basidiobolomycosis in a lung cancer patient. lung cancer Introduction Basidiobolo mycosis is caused by the fungus cases are from Southern part.( Sujatha S et Basidiobolus ranarum, which is a al., 2003; Chandrasekhar HR et al., 1998; zygomycetes belonging to order Prasad PV et al., 2002; Krishnan et al., Entomophthorales. (Gugnani, H. C et al., 1998) Rare dissemination with visceral 1999) This filamentous fungus is usually involvement by Basidiobolus are quoted by associated with subcutaneous zygomycosis various authors such as gastrointestinal tract, of trunk and limbs in immune competent uterus, urinary bladder and retro peritoneum. individuals (Ribes JA et al., 2000) It is an (Bigliazzi C et al., 2004; Khan ZU et al., environmental saprophyte isolated mostly 2001; Nazir Z et al., 1997; Choonhakarn C from decaying vegetation, foodstuffs, fruits, et al., 2004) Pulmonary involvement are and soil. -

Ricardo-La-Hoz-Cv.Pdf
Ricardo M. La Hoz, MD, FACP, FAST Curriculum vitae Date Prepared: November 20th 2017 Name: Ricardo M. La Hoz Office Address: 5323 Harry Hines Blvd Dallas TX, 75390-9113 Work Phone: (214) 648-2163 Work E-Mail: [email protected] Work Fax: (214) 648-9478 Place of Birth: Lima, Peru Education Year Degree Field of Study Institution (Honors) (Thesis advisor for PhDs) 1998 B.Sc. Biology Universidad Peruana Cayetano Heredia 2005 M.D. Medical Doctor Universidad Peruana Cayetano Heredia Postdoctoral Training Year(s) Titles Specialty/Discipline Institution (Lab PI for postdoc research) 2012 - 2013 Chief Fellow, Infectious University of Alabama at Diseases Birmingham 2012 - 2013 Transplant Infectious Diseases University of Alabama at Birmingham 2010 - 2012 Infectious Diseases University of Alabama at Birmingham 2007 - 2010 Internal Medicine University of Alabama at Birmingham Current Licensure and Certification Licensure • State of Texas Medical License, 2014 - Present. • State of North Carolina Medical License, 2013-2014. Inactive. • State of Alabama Medical License, 2009-2013. Inactive. 1 Board and Other Certification • Texas DPA, 2014 - Present. • Diplomate American Board of Internal Medicine, Subspecialty Infectious Diseases. 2012 - Present • Diplomate American Board of Internal Medicine. 2010- Present • Drug Enforcement Administration Certification, 2010 - Present. • State of Alabama Controlled Substance Certification, 2009-2013. • BLS/ACLS Certification, 2008 - Present • Educational Commission for Foreign Medical Graduates Certification. 2006 Honors and Awards Year Name of Honor/Award Awarding Organization 2017 2017 LEAD Capstone Project Finalist - Office of Faculty Diversity & Development, UT Leadership Emerging in Academic Southwestern Medical Center, Dallas, TX. Departments (LEAD) Program for Junior Faculty Physicians and Scientists 2017 2017 Participant - Leadership Emerging in Office of Faculty Diversity & Development, UT Academic Departments (LEAD) Program for Southwestern Medical Center, Dallas, TX. -
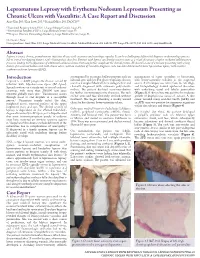
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting As
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting as Chronic Ulcers with Vasculitis: A Case Report and Discussion Anny Xiao, DO,* Erin Lowe, DO,** Richard Miller, DO, FAOCD*** *Traditional Rotating Intern, PGY-1, Largo Medical Center, Largo, FL **Dermatology Resident, PGY-2, Largo Medical Center, Largo, FL ***Program Director, Dermatology Residency, Largo Medical Center, Largo, FL Disclosures: None Correspondence: Anny Xiao, DO; Largo Medical Center, Graduate Medical Education, 201 14th St. SW, Largo, FL 33770; 510-684-4190; [email protected] Abstract Leprosy is a rare, chronic, granulomatous infectious disease with cutaneous and neurologic sequelae. It can be a challenging differential diagnosis in dermatology practice due to several overlapping features with rheumatologic disorders. Patients with leprosy can develop reactive states as a result of immune complex-mediated inflammatory processes, leading to the appearance of additional cutaneous lesions that may further complicate the clinical picture. We describe a case of a woman presenting with a long history of a recurrent bullous rash with chronic ulcers, with an evolution of vasculitic diagnoses, who was later determined to have lepromatous leprosy with reactive erythema nodosum leprosum (ENL). Introduction accompanied by an intense bullous purpuric rash on management of sepsis secondary to bacteremia, Leprosy is a slowly progressive disease caused by bilateral arms and face. For these complaints she was with lower-extremity cellulitis as the suspected infection with Mycobacterium leprae (M. leprae). seen in a Complex Medical Dermatology Clinic and source. A skin biopsy was taken from the left thigh, Spread continues at a steady rate in several endemic clinically diagnosed with cutaneous polyarteritis and histopathology showed epidermal ulceration countries, with more than 200,000 new cases nodosa. -

WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/134709 Al 12 September 2014 (12.09.2014) P O P C T (51) International Patent Classification: (81) Designated States (unless otherwise indicated, for every A61K 31/05 (2006.01) A61P 31/02 (2006.01) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (21) International Application Number: BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, PCT/CA20 14/000 174 DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (22) International Filing Date: HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, 4 March 2014 (04.03.2014) KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (25) Filing Language: English OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, (26) Publication Language: English SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, (30) Priority Data: ZW. 13/790,91 1 8 March 2013 (08.03.2013) US (84) Designated States (unless otherwise indicated, for every (71) Applicant: LABORATOIRE M2 [CA/CA]; 4005-A, rue kind of regional protection available): ARIPO (BW, GH, de la Garlock, Sherbrooke, Quebec J1L 1W9 (CA). GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, SZ, TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, TJ, (72) Inventors: LEMIRE, Gaetan; 6505, rue de la fougere, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, DK, Sherbrooke, Quebec JIN 3W3 (CA). -

Bacterial Infections Diseases Picture Cause Basic Lesion
page: 117 Chapter 6: alphabetical Bacterial infections diseases picture cause basic lesion search contents print last screen viewed back next Bacterial infections diseases Impetigo page: 118 6.1 Impetigo alphabetical Bullous impetigo Bullae with cloudy contents, often surrounded by an erythematous halo. These bullae rupture easily picture and are rapidly replaced by extensive crusty patches. Bullous impetigo is classically caused by Staphylococcus aureus. cause basic lesion Basic Lesions: Bullae; Crusts Causes: Infection search contents print last screen viewed back next Bacterial infections diseases Impetigo page: 119 alphabetical Non-bullous impetigo Erythematous patches covered by a yellowish crust. Lesions are most frequently around the mouth. picture Lesions around the nose are very characteristic and require prolonged treatment. ß-Haemolytic streptococcus is cause most frequently found in this type of impetigo. basic lesion Basic Lesions: Erythematous Macule; Crusts Causes: Infection search contents print last screen viewed back next Bacterial infections diseases Ecthyma page: 120 6.2 Ecthyma alphabetical Slow and gradually deepening ulceration surmounted by a thick crust. The usual site of ecthyma are the legs. After healing there is a permanent scar. The pathogen is picture often a streptococcus. Ecthyma is very common in tropical countries. cause basic lesion Basic Lesions: Crusts; Ulcers Causes: Infection search contents print last screen viewed back next Bacterial infections diseases Folliculitis page: 121 6.3 Folliculitis