Locating and Estimating Sources of Nickel
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Pennsylvania State University Center for Critical Minerals Final
The Pennsylvania State University Center for Critical Minerals Final Report on Cobalt Production in Pennsylvania: Context and Opportunities PREPARED FOR LEONARDO TECHNOLOGIES LLC., PREPARED BY Peter L. Rozelle Feifei Shi Mohammad Rezaee Sarma V. Pisupati Disclaimer This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. ii Executive Summary Cobalt is a critical mineral as defined by the U.S. Department of the Interior in response to Executive Order 13817 (2017). Cobalt metal is used in superalloys required in the hot gas path of stationary gas turbines and jet engines, as well as in samarium cobalt magnets, the domestic production capability of which was found to be essential to the National Defense under Presidential Determination 2019-20. Cobalt compounds are used in lithium-ion batteries, as are manganese compounds. The Defense Industrial Base Report, in response to Executive Order 13806 (2017), expressed concerns regarding the U.S. -

Overview of the Nickel Market in Latin America and the Caribbean
INSG SECRETARIAT BRIEFING PAPER April 2021 – No.35 Overview of the Nickel market in Latin America and the Caribbean Ricardo Ferreira, Director of Market Research and Statistics Francisco Pinto, Manager of Statistical Analysis 1. Introduction At the suggestion of the Group’s Brazilian delegation, it was agreed by members that a report, based on INSG Insight No. 26, published in November 2015 entitled “An overview of the nickel industry in Latin America”, be prepared by the secretariat. This would include an update of the operations that resumed production (e.g. Falcondo in the Dominican Republic), and also discuss how the emerging battery market might influence nickel usage in the region as well as the possibility of nickel scrap usage. This detailed and comprehensive Insight report, the 35th in the series of INSG Insight briefing reports, provides members with the results of that research work. In Latin America and the Caribbean, the nickel producing countries are Brazil, Colombia, Cuba, Dominican Republic, Guatemala and Venezuela. The Dominican Republic stopped nickel mining and smelting in October 2013 but resumed production at the beginning of 2016. Venezuela has not produced since mid-2015. All of these countries mine nickel and process it further to produce intermediate or primary nickel – mainly ferronickel. Most of the mined ore is processed within each country, and then exported to overseas markets, but Brazil and Guatemala also export nickel ore. In terms of usage only Brazil and Mexico are relevant regarding the global nickel market. The first section of this report briefly describes existing nickel deposits in the region. -

The Parallel Oxidation of Carbon and Chromium
THE PARALLEL OXIDATION OF CARBON AND CHROMIUM IN LIQUID IRON (16oo0 c) by LYALL FRANKLIN BARNHARDT B. Sc. Queen's University (1947) Submitted in partial fullfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY at the Massachusetts Institute of Technology 1965 Signature of Author Department of Metallurgy Signature of Professor in Charge of Research jE Signature of Chairman of - A ii Departmental Committee on Graduate Students VI 38 ABSTRACT THE PARALLEL OXIDATION OF CARBON AND CHROMIUM IN LIQUID IRON (16000c) by LYALL FRANKLIN BARNHARDT Submitted to the Department of Metallurgy in April, 1965, in partial fulfillment of the requirements for the Degree of Doctor of Philosophy. The path followed by carbon and chromium concentrations in liquid iron during oxidation was investigated experimentally by blowing gaseous oxygen on to slag-free iron - chromium - carbon melts at 16004C in an induction furnace. The carbon oxidation reaction occurred at the surface of the melt, with an initial constant rate of decarburization which was dependent on the rate of oxygen input. The mechanism of this reaction is expressed in the chemical equation: C + 1/2 02 (g) = CO (g). During the initial stage, which was characterized by a constant rate of decarburization, carbon oxidized preferentially with no loss of chromium. Beyond this stage, the rate of carbon loss declined rapidly and the oxidation of iron and chromium occurred. The entry of argon into the furnace atmosphere, during the oxygen blow, lowered the partial pressure of carbon monoxide at the metal surface. This allowed the carbon concentration of the melt to drop far below the equilibrium iii carbon content for one atmosphere pressure of carbon monoxide. -

Rebel Attacks on Nickel Ore Mines in the Surigao Del Norte Region
Mindanao, Philippines – rebel attacks on nickel ore mines in the Surigao del Norte region The attacks On 3 October 2011, three mining firms located in and around Claver town in the Surigao del Norte region of Mindanao, Philippines were attacked by the rebel group known as the New People’s Army (NPA). It was reported that the attacks were prompted by a refusal of the mining firms to pay a "revolutionary tax" demanded by the NPA. The affected mining firms are the Taganito Mining Corporation (TMC), Taganito HPAL Nickel Corporation and Platinum Group Metals Corporation (PGMC)1. According to news reports and Gard’s local correspondent in the Philippines, some 300 NPA rebels wearing police/military uniforms staged coordinated strikes on the three mining facilities. The rebels attacked TMC’s compound first, burning heavy equipment, service vehicles, barges and buildings. Other rebel groups simultaneously attacked the nearby PGCM and Taganito HPAL compounds. Unconfirmed reports indicate that, unfortunately, casualties were also experienced during the attacks. All foreign vessels anchored near Taganito at the time of the attacks left the anchorage safely. The current situation The Philippine Government has stated that the situation is now contained and has deployed additional troops in the area to improve security and pursue the NPA rebels. However, according to Gard’s local correspondent in the Philippines, threats of further attacks made by the NPA in a press release are believed by many to be real. According to the latest press releases (Reuters on 5 October), Nickel Asia Corporation has resumed operations at its biggest nickel mine TMC and expects to ship ore in the next three weeks. -
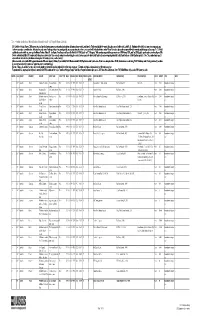
Mineral Facilities of Asia and the Pacific," 2007 (Open-File Report 2010-1254)
Table1.—Attribute data for the map "Mineral Facilities of Asia and the Pacific," 2007 (Open-File Report 2010-1254). [The United States Geological Survey (USGS) surveys international mineral industries to generate statistics on the global production, distribution, and resources of industrial minerals. This directory highlights the economically significant mineral facilities of Asia and the Pacific. Distribution of these facilities is shown on the accompanying map. Each record represents one commodity and one facility type for a single location. Facility types include mines, oil and gas fields, and processing plants such as refineries, smelters, and mills. Facility identification numbers (“Position”) are ordered alphabetically by country, followed by commodity, and then by capacity (descending). The “Year” field establishes the year for which the data were reported in Minerals Yearbook, Volume III – Area Reports: Mineral Industries of Asia and the Pacific. In the “DMS Latitiude” and “DMS Longitude” fields, coordinates are provided in degree-minute-second (DMS) format; “DD Latitude” and “DD Longitude” provide coordinates in decimal degrees (DD). Data were converted from DMS to DD. Coordinates reflect the most precise data available. Where necessary, coordinates are estimated using the nearest city or other administrative district.“Status” indicates the most recent operating status of the facility. Closed facilities are excluded from this report. In the “Notes” field, combined annual capacity represents the total of more facilities, plus additional -

Appendix H EPA Hazardous Waste Law
Appendix H EPA Hazardous Waste Law This Appendix is intended to give you background information on hazardous waste laws and how they apply to you. For most U.S. Environmental Protection Agency (EPA) requirements that apply to the University, the Safety Department maintains compliance through internal inspections, record keeping and proper disposal. In Wisconsin, the Department of Natural Resources (DNR) has adopted the EPA regulations, consequently EPA and DNR regulations are nearly identical. EPA defines This Appendix only deals with "hazardous waste" as defined by the EPA. hazardous waste as Legally, EPA defines hazardous waste as certain hazardous chemical waste. This hazardous chemical Appendix does not address other types of regulated laboratory wastes, such as waste; radioactive, infectious, biological, radioactive or sharps. Chapter 8 descibes disposal procedures infectious and biohazardous waste for animals. Chapter 9 describes disposal procedures for sharps and other waste that are regulated by can puncture tissue. Chapter 11 discusses Radiation and the Radiation Safety for other agencies. Radiation Workers provides guidelines for the disposal of radioactive waste. Procedures for medical waste are written by the UW Hospital Safety Officer. The Office of Biological Safety can provide guidance for the disposal of infectious and biological waste. EPA regulations focus on industrial waste streams. As a result, many laboratory chemical wastes are not regulated by EPA as hazardous chemical waste. However, many unregulated chemical wastes do merit special handling and disposal If a waste can be procedures. Thus, Chapter 7 and Appendix A of this Guide recommend disposal defined as: procedures for many unregulated wastes as if they were EPA hazardous waste. -

Corrosion Resistance of Nickel and Nickel- Containing Alloys in Caustic Soda and Other Alkalies (Ceb-2)
CORROSION RESISTANCE OF NICKEL AND NICKEL- CONTAINING ALLOYS IN CAUSTIC SODA AND OTHER ALKALIES (CEB-2) A PRACTICAL GUIDE TO THE USE OF NICKEL-CONTAINING ALLOYS NO 281 Distributed by Produced by NICKEL INCO INSTITUTE CORROSION RESISTANCE OF NICKEL AND NICKEL-CONTAINING ALLOYS IN CAUSTIC SODA AND OTHER ALKALIES (CEB-2) A PRACTICAL GUIDE TO THE USE OF NICKEL-CONTAINING ALLOYS NO 281 Originally, this handbook was published in 1973 by INCO, The International Nickel Company, Inc. Today this company is part of Vale S.A. The Nickel Institute republished the handbook in 2020. Despite the age of this publication the information herein is considered to be generally valid. Material presented in the handbook has been prepared for the general information of the reader and should not be used or relied on for specific applications without first securing competent advice. The Nickel Institute, the American Iron and Steel Institute, their members, staff and consultants do not represent or warrant its suitability for any general or specific use and assume no liability or responsibility of any kind in connection with the information herein. Nickel Institute [email protected] www.nickelinstitute.org Table of Contents Page PART I. INTRODUCTION 3 PART II. CORROSION BY CAUSTIC SODA........................... 4 A. Nickel .................................... _ . 4 1. Effect of Concentration, Temperature and Carbon Content ...... _ . 4 2. Effect of Velocity ............... _ ... __ ..................... _ 6 3. Effect of Aeration .•......................................... 6 4. Effect of System Thermal Gradients .............................. 7 5. Effect of Impurities .,. _................. _ ............ _ . 7 6. Effect of Stress ............................................. 8 7. Effect of Dissimilar Metal Contact .. _ ...... _ ..... -

Nickel Carbonyl Final AEGL Document
Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 6 Committee on Acute Exposure Guideline Levels, Committee on Toxicology, National Research Council ISBN: 0-309-11214-1, 318 pages, 6 x 9, (2007) This free PDF was downloaded from: http://www.nap.edu/catalog/12018.html Visit the National Academies Press online, the authoritative source for all books from the National Academy of Sciences, the National Academy of Engineering, the Institute of Medicine, and the National Research Council: • Download hundreds of free books in PDF • Read thousands of books online, free • Sign up to be notified when new books are published • Purchase printed books • Purchase PDFs • Explore with our innovative research tools Thank you for downloading this free PDF. If you have comments, questions or just want more information about the books published by the National Academies Press, you may contact our customer service department toll-free at 888-624-8373, visit us online, or send an email to [email protected]. This free book plus thousands more books are available at http://www.nap.edu. Copyright © National Academy of Sciences. Permission is granted for this material to be shared for noncommercial, educational purposes, provided that this notice appears on the reproduced materials, the Web address of the online, full authoritative version is retained, and copies are not altered. To disseminate otherwise or to republish requires written permission from the National Academies Press. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 6 http://www.nap.edu/catalog/12018.html Committee on Acute Exposure Guideline Levels Committee on Toxicology Board on Environmental Studies and Toxicology Copyright © National Academy of Sciences. -

Böhler Edelstahl - General Information
Content . Böhler Edelstahl - General Information . Production Possibilities & New Additive Manufacturing . Product Portfolio Böhler Edelstahl - General Information MISSION We develop, produce and supply high-speed steels, tool steels and special materials for our worldwide customers to offer them optimal solutions. BÖHLER Edelstahl at a glance Worldwide 2016 Production 79 €622 137,000 points of sale million revenue tonnes Advanced 2114 production facilities employees Quality & technology Global 250 LEADER steel grades 100% recyclable Sales revenue by region (FY 2016) 1% 9% 12% Europe America Asia Rest of world 78% Sales revenue by business area (FY 2016) 11% Special materials 41% 21% Tool steel High speed steels Other 27% Business area revenue special materials Automotive Other Engineering 4% 11% 11% Special Oil & Gas, materials CPI High speed Power-Gen steel 21% 41% 43% 15% 27% Tool steel 27% Aerospace Production Possibilities BÖHLER EDELSTAHL Integrated plant configuration Whole melting and re-melting operations at one site Whole hot forming operations at one site Whole heat treatment operations at one site Whole ND-Testing and finishing operations at one site Standard mechanical, metallographic, chemical tests at one site Quality starts here Primary metallurgy EAF/50 t Electric arc furnace VIM Vacuum induction furnace AOD Argon oxygen decarburization VID/14 t Vacuum induction furnace REMELTING Remelting process for high- purity metallurgical materials • ESR • PESR • VAR FORGING TECHNOLOGY 5,200 t forging press Open die forge Bar steel -

Safety Data Sheet According to Regulation (EC) No. 1907/2006 (REACH) As Amended Material Name: Carbon Dioxide (DX) Gas Purifier Media SDS ID: 0048 (EU)
Safety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH) as amended Material Name: Carbon Dioxide (DX) Gas Purifier Media SDS ID: 0048 (EU) SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Material Name Carbon Dioxide (DX) Gas Purifier Media Product Code 8008786 Product Description The media contained in this product, when used as designed, under normal operating conditions, and installed and maintained according to product literature, is not expected to be hazardous. Classifications and hazards represented on this Safety Data Sheet are only applicable in the unlikely event that the purifier media is liberated from the purifier housing. The purifier has sieves internal to the housing to prevent the media from escaping during intended use. Caution: Some units are provided with a fill port, which is factory sealed with a VCR® fitting and plug, covered with red shrink wrap. This port must never be opened by the end user, since it will potentially result in a release of the media. Registration status REACH compliance status of the substance is currently under investigation. 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses For removal of volatile acids and bases, refractory compounds, condensable organics, non-condensable organics and moisture from carbon dioxide gas Uses advised against Use only with gases listed in Identified Uses. 1.3 Details of the supplier of the safety data sheet Entegris GmbH Hugo-Junkers-Ring 5, Gebäude 107/W, 01109 Dresden, Germany Telephone Number: +49 (0) 351 795 97 0 Fax Number: +49 (0) 351 795 97 499 Only Representative Tetra Tech International, Inc. -

Method for Boriding of Coatings Using High Speed
(19) & (11) EP 2 222 898 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C25D 9/08 (2006.01) C23C 28/00 (2006.01) 08.06.2011 Bulletin 2011/23 C25D 3/66 (2006.01) C25D 9/04 (2006.01) (21) Application number: 08848442.3 (86) International application number: PCT/EP2008/065065 (22) Date of filing: 06.11.2008 (87) International publication number: WO 2009/060033 (14.05.2009 Gazette 2009/20) (54) METHOD FOR BORIDING OF COATINGS USING HIGH SPEED ELECTROLYTIC PROCESS VERFAHREN ZUM BORIEREN VON BESCHICHTUNGEN MIT HILFE EINES SCHNELLEN ELEKTROLYTISCHEN VERFAHRENS PROCÉDÉ DE BORURATION DE REVÊTEMENTS À L’AIDE D’UN TRAITEMENT ÉLECTROLYTIQUE HAUTE VITESSE (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Ürgen, Mustafa K. HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT Istanbul (TR) RO SE SI SK TR • Timur, Servet I. Istanbul (TR) (30) Priority: 09.11.2007 EP 07120419 • Kazmanli, Kürsat M. Istanbul (TR) (43) Date of publication of application: • Kartal, Güldem 01.09.2010 Bulletin 2010/35 Istanbul (TR) (73) Proprietors: (74) Representative: Sevinç, Erkan • Ürgen, Mustafa K. Istanbul Patent & Trademark Consultancy Ltd. Istanbul (TR) Plaza-33, Büyükdere cad. No: 33/16 • Timur, Servet I. Sisli Istanbul (TR) 34381 Istanbul (TR) • Kazmanli, Kürsat M. Istanbul (TR) (56) References cited: • Kartal, Güldem DE-A1- 1 796 215 GB-A- 1 422 859 Istanbul (TR) JP-A- 5 161 090 US-A- 3 824 134 Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

A Pilgrimage Into the Archives of Nickel Toxicology
ANNALS OF CLINICAL AND LABORATORY SCIENCE, Vol. 19, No. 1 Copyright © 1989, Institute for Clinical Science, Inc. A Pilgrimage into the Archives of Nickel Toxicology F. WILLIAM SUNDERMAN, M.D., Ph .D. Institute for Clinical Science, Pennsylvania Hospital,. Philadelphia, PA 19107 Introduction the contributors to this and the three previous symposia, with special com It is my great pleasure to address this mendation to my beloved son, Bill Jr. assembly of learned scientists on the My memory with respect to nickel tox occasion of the Fourth International icology goes back to 1943 when I Conference on Nickel Metabolism and attended my first nickel meeting at Los Toxicology. Alamos, New Mexico. This initial meet When I seriously sat down to prepare ing was arranged out of necessity. It was remarks for this evening’s program, I held for the purpose of devising methods became aghast as I pondered upon the to protect workers in nuclear energy title that I had submitted. How could I from the hazards of acute and chronic presume to cover such a colossal subject exposure to nickel tetracarbonyl in the course of a brief address? It is true Ni(CO)4. It was recognized then that that the hazards of exposure to nickel nickel carbonyl was one of the most toxic and nickel compounds have been recog of all gases. At our first meeting, those in nized only within recent decades; how attendance set three goals: (1 ) to obtain ever, the number of research contribu accurate toxicity data on nickel carbonyl; tions and publications on nickel (2 ) to establish programs for the protec toxicology that have appeared during the tion and treatment of workers who might past three decades has been substantial.