Superseded Marianne Peikert
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dock and Crop Images
orders: [email protected] (un)subscribe: [email protected] Current Availability for September 25, 2021 Dock and Crop images Click any thumbnail below for the slideshow of what we shipped this past week: CYCS ARE RED HOT GIANT GLOSSY LEAVES BLUE MOONSCAPE SUCCULENT BLUE LEAVES SUCCULENT ORANGE LEAVES SPECKLED LEAVES CYCS ARE RED HOT RED SUNSETSCAPE Jeff's updates - 9/16 dedicated this week's favorites Chimi's favorite climbing structure 4FL = 4" pot, 15 per flat 10H = 10" hanging basket n = new to the list ys = young stock 6FL = 6" pot, 6 per flat 10DP = 10" Deco Pot, round b&b = bud and bloom few = grab 'em! QT= quart pot, 12 or 16 per flat nb = no bloom * = nice ** = very nice Quarts - 12 per flat, Four Inch - 15 per flat, no split flats, all prices NET code size name comments comments 19406 4FL Acalypha wilkesiana 'Bronze Pink' ** Copper Plant-colorful lvs 12210 QT Acorus gramineus 'Ogon' ** lvs striped creamy yellow 19069 4FL Actiniopteris australis ** Eyelash Fern, Ray Fern 17748 4FL Adiantum hispidulum ** Rosy Maidenhair 17002 4FL Adiantum raddianum 'Microphyllum' ** extremely tiny leaflets 21496 4FL Adromischus filicaulis (cristatus?) ** Crinkle Leaf 16514 4FL Aeonium 'Kiwi' ** tricolor leaves 13632 QT Ajuga 'Catlin's Giant' ** huge lvs, purple fls 13279 QT Ajuga pyramidalis 'Metallica Crispa' ** crinkled leaf 17560 4FL Aloe vera * Healing Aloe, a must-have 13232 QT Anthericum sanderii 'Variegated' *b&b grassy perennial 13227 QT Asparagus densiflorus 'Meyer's' ** Foxtail Fern 19161 4FL Asplenium 'Austral Gem' -
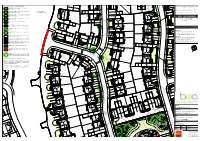
PLANNING NHBC 8 No
© Crown Copyright and Database Right This drawing and the design it depicts are copyright and may not be copied alt: or reproduced without written permission from Bea Landscape Design Ltd. No liability will be accepted for amendments made by others. This drawing is to DP 4 No. Lonicera nitida 'Baggesen's Gold' be read in conjunction with the landscape specification and other relevant DP 3 No. Viburnum davidiidrawings. 5 No. Rosmarinus officinalis © Crown Copyright. All rights reserved 100018739. 54 No. Photinia x fraseri 'Little Red Robin'Contains Ordnance Survey Data : DP 2019. Do not scale from this drawing. Figured dimensions only to be used. Refer any query to office of origination. NHBC date: amendments NHBC rev as instructed A 01.04.2020 to co-ord with revised layout NHBC B 04.09.2020 to co-ord with revised layout Corylus colurnaNHBC 14-16cm C 15.10.2020 to co-ord with revised layout / 107 NHBC NHBC NHBC 4 No. Cotoneaster suecicusNHBC 'Skogholm' 10-12L 19 No. Photinia x fraseri 'Little Red Robin' NHBC NHBC 4 No. Viburnum davidiiNHBC 10-12L 5 No. Carex pendula 8 No. Hedera helix 'Glacier' 3L 5 No. Carex oshimensis 'Evergold' 3 No. Ceanothus thyrsiflorus repens 3 No. Brachyglottis cdm'Sunshine' 2015; designers residual risk information 3 No. Cistus 'Grayswood Pink' 20 No. Photinia x fraseriIn addition 'Little to Red the risksRobin' & hazards normally associated with the type of work detailed 5-7.5L 108 on this drawing the following residual risks should be noted: 10-12L 109 Construction: Lavandula angustifolia 'Loddon Pink' 5-7.5L 4 No. -

Journal Editorial Staff: Rachel Cobb, David Pfaff, Patricia Riley Hammer, Henri Nier, Suzanne Pierot, Sabina Sulgrove, Russell Windle
Spring 2010 Volume 36 IVY J OURNAL IVY OF THE YEAR 2011 Hedera helix ‘Ivalace’ General Information Press Information American Ivy Society [email protected] P. O. Box 163 Deerfield, NJ 08313 Ivy Identification, Registration Membership Russell A. Windle The American Ivy Society Membership American Ivy Society Laurie Perper P.O. Box 461 512 Waterford Road Lionville, PA 19353-0461 Silver Spring, MD, 20901 [email protected] Officers and Directors President—Suzanne Warner Pierot Treasurer—Susan Hendley Membership—Laurie Perper Registrar, Ivy Research Center Director—Russell Windle Taxonomist—Dr. Sabina Mueller Sulgrove Rosa Capps, Rachel Cobb, Susan Cummings, Barbara Furlong, Patricia Riley Hammer, Constance L. Meck, Dorothy Rouse, Daphne Pfaff, Pearl Wong Ivy Journal Editorial Staff: Rachel Cobb, David Pfaff, Patricia Riley Hammer, Henri Nier, Suzanne Pierot, Sabina Sulgrove, Russell Windle The Ivy Journal is published once per year by the American Ivy Society, a nonprofit educational organization. Membership includes a new ivy plant each year, subscription to the Ivy Journal and Between the Vines, the newsletter of The American Ivy Society. Editorial submissions are welcome. Mail typed, double-spaced manuscript to the Ivy Journal Editor, The American Ivy Society. Enclose a self-addressed, stamped envelope if you wish manuscript and/ or artwork to be returned. Manuscripts will be handled with reasonable care. However, AIS assumes no responsibility for safety of artwork, photographs, or manuscripts. Every precaution is taken to ensure accuracy but AIS cannot accept responsibility for the corrections or accuracy of the information supplied herein or for any opinion expressed. The American Ivy Society P. O. Box 163, Deerfield Street, NJ 08313 www.ivy.org Remember to send AIS your new address. -

Araliaceae – Ginseng Family
ARALIACEAE – GINSENG FAMILY Plant: some herbs (perennial), woody vines, shrubs and trees Stem: usually pithy Root: sometimes with rhizomes Leaves: simple or palmately compound but rarely 2’s or 3’s, often thickened and large, mostly alternate (rarely opposite or whorled); usually with stipules that forms a stem sheath; often with star-shaped hairs Flowers: mostly perfect or unisexual (monoecious or dioecious), regular (actinomorphic); flowers very small, mostly in umbels; sepals 5, often forming small teeth or none, mostly 5(-10) petals; mostly 5(-10) stamens; ovary inferior, 2-5 (10) fused carpels Fruit: berry or drupe, oily Other: mostly tropical and subtropical, a few oranamentals; similar to Apiaceae; Dicotyledons Group Genera: 70+ genera; locally Aralia (spikenard), Hedera (English Ivy), Oplopanax, Panax (ginseng) WARNING – family descriptions are only a layman’s guide and should not be used as definitive Araliaceae (Ginseng Family) – 5 (mostly) sepals and petals (often 5-lobed), often in umbels or compound umbels; leaves simple or more often compound; fruit a berry or drupe Examples of common genera Devil's Walkingstick [Hercules’ Club] Wild Sarsaparilla Aralia spinosa L. Aralia nudicaulis L. Devil's Club [Devil’s Walking Stick; Alaskan Ginseng] Oplopanax horridus (Sm.) Miq. English Ivy Hedera helix L. (Introduced) Dwarf Ginseng Panax trifolius L. ARALIACEAE – GINSENG FAMILY Wild Sarsaparilla; Aralia nudicaulis L. Devil's Walkingstick [Hercules’ Club]; Aralia spinosa L. English Ivy; Hedera helix L. (Introduced) Devil's Club [Devil’s -

Greenery Planning for Improvement of Urban Air Quality—A Review †
Proceedings Greenery Planning for Improvement of Urban Air Quality—A Review † Kamil Leszek Rawski Faculty of Civil and Environmental Engineering, Bialystok University of Technology, Wiejska 45E, 15-351 Bialystok, Poland; [email protected]; Tel.: +48-727-900-201 † Presented at the Innovations-Sustainability-Modernity-Openness Conference (ISMO’19), Bialystok, Poland, 22–23 May 2019. Published: 12 June 2019 Abstract: On the basis of Polish and foreign literature on the subject, the impact of vegetation on air pollution (e.g., particulate matter) was described, as well as what significance its proper arrangement has. Reviewing findings carried out by various researchers, the criteria of selecting plants were collected and specified. Only those criteria that contribute to obtaining optimal results in the fight against air pollution were taken into consideration. Also, based on the collected data, a set of guidelines was developed that could eventually serve as a tool for more effective planning of urban greenery. Keywords: urban greenery; planning; trees; shrubs; air pollution; particulate matter; phytoremediation 1. Introduction Along with the continuous process of urbanization, standards of air purity are increasingly being exceeded, and smog is becoming an increasingly common phenomenon. Currently, constant actions are being undertaken to change this situation by limiting both current and future pollutant emissions. The first solution can be facilitated by using various methods. One of these methods is the phytoremediation method, i.e. using plants. However, in order to make this process optimal, it is also necessary to plan vegetation in the cities in an appropriate way. This is because plant arrangements in urbanized areas can affect the flow of air and this has direct influence on its quality. -

Westport Headlands Invasive Vegetation Management
Westport Headlands Invasive Vegetation Management Priority Species (see map) English Ivy Hedera helix Cape Ivy Delairea odorata Periwinkle Vinca major Cotoneaster Cotoneaster lacteus Pampas grass Cortaderia jubata Wild radish Raphanus sativus Mowed Areas • Native plants present and should be avoided if possible. • Potential to plant natives in cleared areas and should be avoided by mowing activities; if natives are planted, an area should be completely cleared of invasives within a 3-foot radius and clearly marked to avoid destruction. • Disking should occur in already mowed areas followed by hand removal of roots/vegetation • Mowing should occur four times a year to prevent further seed production, stimulate seed germination, and starve rhizomes/roots. • Quarterly hand removal efforts should be conducted once invasive species have been sufficiently reduced. Scraped Areas • Follow-up with disking to loosen underground material and follow-up with hand removal of all material possible as soon as possible. This area should be mowed four times a year following this initial effort. • Once invasive species have been sufficiently reduced (likely a couple years away), quarterly hand removal efforts should be conducted. • If feasible, native plantings may be placed in locations which may be avoided by future mowing/removal/control efforts; areas planted with natives should be cleared of all invasive species within a 3-foot buffer and clearly marked to avoid destruction. Cultural Sites • No ground disturbance allowed and will likely be continuous source of propagules for invasives into other areas on the headlands. • Strong initial control measures using mowing (if allowed), weed-wacker and hand tools used to cut vegetation to ground level. -

Proper Listing of Scientific and Common Plant Names In
PROPER USAGE OF PLANT NAMES IN PUBLICATIONS A Guide for Writers and Editors Kathy Musial, Huntington Botanical Gardens, August 2017 Scientific names (also known as “Latin names”, “botanical names”) A unit of biological classification is called a “taxon” (plural, “taxa”). This is defined as a taxonomic group of any rank, e.g. genus, species, subspecies, variety. To allow scientists and others to clearly communicate with each other, taxa have names consisting of Latin words. These words may be derived from languages other than Latin, in which case they are referred to as “latinized”. A species name consists of two words: the genus name followed by a second name (called the specific epithet) unique to that species; e.g. Hedera helix. Once the name has been mentioned in text, the genus name may be abbreviated in any immediately subsequent listings of the same species, or other species of the same genus, e.g. Hedera helix, H. canariensis. The first letter of the genus name is always upper case and the first letter of the specific epithet is always lower case. Latin genus and species names should always be italicized when they appear in text that is in roman type; conversely, these Latin names should be in roman type when they appear in italicized text. Names of suprageneric taxa (above the genus level, e.g. families, Asteraceae, etc.), are never italicized when they appear in roman text. The first letter of these names is always upper case. Subspecific taxa (subspecies, variety, forma) have a third epithet that is always separated from the specific epithet by the rank designation “var.”, “ssp.” or “subsp.”, or “forma” (sometimes abbreviated as “f.”); e.g. -

STRAW Project 2014‐2015 Restoration Report: Miller Creek
STRAW Project 2014‐2015 Restoration Report: Miller Creek PROJECT DESCRIPTION Students and Teachers Restoring A Watershed (STRAW) continues to work on riparian vegetation restoration along Miller Creek near Miller Creek Middle School under contract with the Marin County Stormwater Pollution Prevention Program (MCSTOPPP). The focus has been the removal of invasive exotic plant species, planting native species, erosion control, and providing educational and environmental stewardship opportunities for the local school. PROJECT PARTICIPANTS School Number of Restoration Number of Name Teacher Grade Classroom Date Students/ Presentations volunteers Mike Schulist 8th ‐ 11/17/14 183 Miller Janice Woods 8th ‐ Creek Bob Arigi 6th 5 11/24/14 234 Middle Erik Lunde 6th 4 School Sue Holland 7th ‐ 03/03/15 200 Erik Lunde 7th ‐ TOTALS 5 ‐ 9 3 617 WORK COMPLETED The following work completed includes weeding and planting in zones designated on the site sketch revised October 2014 (see attached document, “Miller CR 2014.pdf”). The main invasive plant species removed were English ivy (Hedera helix), Himalayan blackberry (Rubus armeniacus), and cape ivy (Delairea odorata). Basket sedge (Carex barbarae) was transplanted from the large patch upstream of the areas of removal. Approximately 145 sedge transplants were installed in the work zones as described below. Eight container shrubs were purchased, but not installed because…. These will be installed next year. Table 1 summarizes total amount of invasive species removed and area restored. Zone 1.12 Clearing and sedge planting has been largely successful here along a small drainage swale. Elderberries planted in 2013‐2014 are performing well at the toe of the upper slope. -

Wallcreeper Break
Wallcreeper and woodpecker break (Istria) 9 – 13 March 2016 Participants Mike Kempton and John Lerpiniere Karen Foulkes John and Sarah Barney Leader Paul Tout Report by Paul Tout. Photos by Mike Kempton (MK) and Paul Tout (PT), all taken on the holiday. Cover: crested tit, grey-headed woodpecker and wallcreeper (all MK). Below: photo of the group. We stayed at Hotel Mirna at the spa of Istarske Toplice in Istria. This holiday, as for every Honeyguide holiday, also puts something into conservation in our host country by way of a contribution to the wildlife that we enjoyed. The conservation contribution of £40 per person was supplemented by Gift Aid and we were able to give £250 to DOPPS (BirdLife Slovenia). As at the end of March 2016, the total for all conservation contributions through Honeyguide since 1991 was £105,338. Above: Paul presents the DOPPS representative, Borut Mozetič, with the donation; see account on page 7. 2 9 March – Arrival at airport and travel to our hotel. The planes (from Munich and Stansted) arrived at Trieste on time. For Karen and Paul there was time to check the airport grounds and three birds of prey were noted to the list, hen harrier (2), common buzzard and Eurasian kestrel, and soon we were off towards our hotel in NW Croatia about 90 minutes away. Everything went smoothly along the coast and through Trieste and by 8:00 p.m. we were sitting in the restaurant and enjoying two magnificent home-made ‘fresh’ pastas, one with white truffles Tuber magnatum and the other with penny-buns Boletus edulis, the latter being extraordinarily abundant in Istria’s oakwoods in autumn 2015. -

Hedera Helix Information Guide
New York City EcoFlora Hedera helix L. English Ivy Araliaceae (Aralia family) Description: Evergreen woody vine, young shoots and inflorescences covered with stellate hairs or peltate scales; juvenile stems creeping and forming extensive mats, the mature stems up to 25 centimeters diameter, climbing by abundant, adventitious roots with adhesive pads. Leaves simple, alternate, dimorphic, 4–10 cm long; juvenile leaves palmately lobed with 3 or 5 triangular lobes, glabrous, the base cordate, the apex acute or acuminate, the upper surface shining and dark green with whitened veins, sometimes tinged purple in winter, the lower surface pale green; mature leaves ovate or rhombic, entire, otherwise like juveniles. Flowers in axillary umbels; sepals 5, very short; petals 5, fleshy, green; stamens 5; ovary inferior. Fruit a drupe with 2–3 pyrennes, turning black at maturity. Where Found: Hedera helix is native to Europe from Ireland and Norway to the Caucasus. In New York City, the species is most abundant near home sites and gardens where there is a history of cultivation, especially in suburban areas. Natural History: In its native Europe, bees and other insects obtain nectar from the flowers and birds disperse the fruit. The adventitious climbing roots secrete an adhesive polymer composed of nanoparticles. Root hairs shrink and curl as they dry, pulling the stem closer to the surface. These forces enable the plant to climb very high and accumulate great mass. When a tree is so encumbered, its photosynthetic potential is diminished and the tree is vulnerable to breakage in wind and ice storms. The ground covering impedes absorption from rainfall and creates desert-like conditions dominated by one species. -

Hedera Helix1
Fact Sheet FPS-239 October, 1999 Hedera helix1 Edward F. Gilman2 Introduction The small, neat foliage of English Ivy provides excellent material for use as a ground cover or can be allowed to climb, its aerial roots firmly attaching to any rough-textured surface (Fig. 1). The dark green leaves are produced in abundance along the rapidly growing but rarely branching stems. It makes a wonderful ground cover giving a gentle uniformity to any landscape. As the plant climbs a wall or other structure and becomes older, mature leaves develop with fewer lobes. General Information Scientific name: Hedera helix Pronunciation: HED-dur-uh HEL-licks Common name(s): English Ivy Family: Araliaceae Plant type: ground cover USDA hardiness zones: 5 through 9 (Fig. 2) Planting month for zone 7: year round Planting month for zone 8: year round Planting month for zone 9: year round Figure 1. English Ivy. Origin: not native to North America Uses: mass planting; container or above-ground planter; Plant habit: prostrate (flat) hanging basket; suitable for growing indoors; cut foliage/twigs Plant density: dense Availablity: generally available in many areas within its Growth rate: fast hardiness range Texture: medium Description Foliage Height: depends upon supporting structure Spread: depends upon supporting structure Leaf arrangement: alternate 1.This document is Fact Sheet FPS-239, one of a series of the Environmental Horticulture Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida. Publication date: October, 1999 Please visit the EDIS Web site at http://edis.ifas.ufl.edu. 2. Edward F. Gilman, professor, Environmental Horticulture Department, Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida, Gainesville, 32611. -

IHCA Recommended Plant List
Residential Architectural Review Committee Recommended Plant List Plant Materials The following plant materials are intended to guide tree and shrub ADDITIONS to residential landscapes at Issaquah Highlands. Lot sizes, shade, wind and other factors place size and growth constraints on plants, especially trees, which are suitable for addition to existing landscapes. Other plant materials may be considered that have these characteristics and similar maintenance requirements. Additional species and varieties may be selected if authorized by the Issaquah Highlands Architectural Review Committee. This list is not exhaustive but does cover most of the “good doers” for Issaquah Highlands. Our microclimate is colder and harsher than those closer to Puget Sound. Plants not listed should be used with caution if their performance has not been observed at Issaquah Highlands. * Drought-tolerant plant ** Requires well-drained soil DECIDUOUS TREES: Small • Acer circinatum – Vine Maple • Acer griseum – Paperbark Maple • *Acer ginnala – Amur Maple • Oxydendrum arboreum – Sourwood • Acer palmation – Japanese Maple • *Prunus cerasifera var. – Purple Leaf Plum varieties • Amelanchier var. – Serviceberry varieties • Styrax japonicus – Japanese Snowbell • Cornus species, esp. kousa Medium • Acer rufinerve – Redvein Maple • Cornus florida (flowering dogwood) • *Acer pseudoplatanus – Sycamore Maple • Acer palmatum (Japanese maple, many) • • *Carpinus betulus – European Hornbeam Stewartia species (several) • *Parrotia persica – Persian Parrotia Columnar Narrow