Maternal and Perinatal Health Research
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Class-8 New 2020.CDR
Class - VIII AGRICULTURE OF ASSAM Agriculture forms the backbone of the economy of Assam. About 65 % of the total working force is engaged in agriculture and allied activities. It is observed that about half of the total income of the state of Assam comes from the agricultural sector. Fig 2.1: Pictures showing agricultural practices in Assam MAIN FEATURES OF AGRICULTURE Assam has a mere 2.4 % of the land area of India, yet supports more than 2.6 % of the population of India. The physical features including soil, rainfall and temperature in Assam in general are suitable for cultivation of paddy crops which occupies 65 % of the total cropped area. The other crops are wheat, pulses and oil seeds. Major cash crops are tea, jute, sugarcane, mesta and horticulture crops. Some of the crops like rice, wheat, oil seeds, tea , fruits etc provide raw material for some local industries such as rice milling, flour milling, oil pressing, tea manufacturing, jute industry and fruit preservation and canning industries.. Thus agriculture provides livelihood to a large population of Assam. AGRICULTURE AND LAND USE For the purpose of land utilization, the areas of Assam are divided under ten headings namely forest, land put to non-agricultural uses, barren and uncultivable land, permanent pastures and other grazing land, cultivable waste land, current fallow, other than current fallow net sown area and area sown more than once. 72 Fig 2.2: Major crops and their distribution The state is delineated into six broad agro-climatic regions namely upper north bank Brahmaputra valley, upper south bank Brahmaputra valley, Central Assam valley, Lower Assam valley, Barak plain and the hilly region. -

Setting Unit-15Assam and Freedom Struggle-II.Pmd
Assam and Freedom Struggle-II Unit 15 UNIT 15:ASSAM AND FREEDOM STRUGGLE-II UNIT STRUCTURE 15.1 Learning Objectives 15.2 Introduction 15.3 Assam and the Civil Disobedience Movement 15.4 The Quit India Movement in Assam 15.5 Grouping Controversy and Independence 15.6 Let Us Sum Up 15.7 Further Reading 15.8 Answers to Check Your Progress 15.9 Model Questions 15.1 LEARNING OBJECTIVES After going through this unit, you will be able to l describe the role of Assam in the Civil Disobedience Movement, l discuss the Quit India Movement and its impact on Assam. 15.2 INTRODUCTION In the previous unit, you have learnt about the non-cooperation movement and its impact on Assam. Now in this unit we shall discuss the last part of the freedom struggle in Assam. 15.3 ASSAM AND THE CIVIL DISOBEDIENCE MOVEMENT The pace of the Indian National movement became slow after the suspension of the Non Co-operation Movement and was revived only with the beginning of the Civil Disobedience Movement. Various developments in the meantime, however, served as the background for the upsurge of the Civil Disobedience Movement. First, the appointment of the Simon Commission in 1927 offered an opportunity to unite the different groups and parties in the country against the British. Second, the freedom movement History of Assam from the 17th Century till 1947 C.E. 193 Unit 15 Assam and Freedom Struggle-II reached new heights centering upon the opposition to the Simon Commission. Protest demonstrations, hartals (strike), etc, were held all over the country when the members of the Commission landed in Bombay. -

Empire's Garden: Assam and the Making of India
A book in the series Radical Perspectives a radical history review book series Series editors: Daniel J. Walkowitz, New York University Barbara Weinstein, New York University History, as radical historians have long observed, cannot be severed from authorial subjectivity, indeed from politics. Political concerns animate the questions we ask, the subjects on which we write. For over thirty years the Radical History Review has led in nurturing and advancing politically engaged historical research. Radical Perspec- tives seeks to further the journal’s mission: any author wishing to be in the series makes a self-conscious decision to associate her or his work with a radical perspective. To be sure, many of us are currently struggling with the issue of what it means to be a radical historian in the early twenty-first century, and this series is intended to provide some signposts for what we would judge to be radical history. It will o√er innovative ways of telling stories from multiple perspectives; comparative, transnational, and global histories that transcend con- ventional boundaries of region and nation; works that elaborate on the implications of the postcolonial move to ‘‘provincialize Eu- rope’’; studies of the public in and of the past, including those that consider the commodification of the past; histories that explore the intersection of identities such as gender, race, class and sexuality with an eye to their political implications and complications. Above all, this book series seeks to create an important intellectual space and discursive community to explore the very issue of what con- stitutes radical history. Within this context, some of the books pub- lished in the series may privilege alternative and oppositional politi- cal cultures, but all will be concerned with the way power is con- stituted, contested, used, and abused. -

History of North East India (1228 to 1947)
HISTORY OF NORTH EAST INDIA (1228 TO 1947) BA [History] First Year RAJIV GANDHI UNIVERSITY Arunachal Pradesh, INDIA - 791 112 BOARD OF STUDIES 1. Dr. A R Parhi, Head Chairman Department of English Rajiv Gandhi University 2. ************* Member 3. **************** Member 4. Dr. Ashan Riddi, Director, IDE Member Secretary Copyright © Reserved, 2016 All rights reserved. No part of this publication which is material protected by this copyright notice may be reproduced or transmitted or utilized or stored in any form or by any means now known or hereinafter invented, electronic, digital or mechanical, including photocopying, scanning, recording or by any information storage or retrieval system, without prior written permission from the Publisher. “Information contained in this book has been published by Vikas Publishing House Pvt. Ltd. and has been obtained by its Authors from sources believed to be reliable and are correct to the best of their knowledge. However, IDE—Rajiv Gandhi University, the publishers and its Authors shall be in no event be liable for any errors, omissions or damages arising out of use of this information and specifically disclaim any implied warranties or merchantability or fitness for any particular use” Vikas® is the registered trademark of Vikas® Publishing House Pvt. Ltd. VIKAS® PUBLISHING HOUSE PVT LTD E-28, Sector-8, Noida - 201301 (UP) Phone: 0120-4078900 Fax: 0120-4078999 Regd. Office: 7361, Ravindra Mansion, Ram Nagar, New Delhi – 110 055 Website: www.vikaspublishing.com Email: [email protected] About the University Rajiv Gandhi University (formerly Arunachal University) is a premier institution for higher education in the state of Arunachal Pradesh and has completed twenty-five years of its existence. -

Government of India Ministry of Health and Family Welfare Department of Health and Family Welfare Lok Sabha Unstarred Question No
GOVERNMENT OF INDIA MINISTRY OF HEALTH AND FAMILY WELFARE DEPARTMENT OF HEALTH AND FAMILY WELFARE LOK SABHA UNSTARRED QUESTION NO. 60 TO BE ANSWERED ON 2ND FEBRUARY, 2018 AMRIT PHARMACIES 60. SHRI NALIN KUMAR KATEEL: Will the Minister of HEALTH AND FAMILY WELFARE be pleased to state: (a) whether the Government has set up Affordable Medicines and Reliable Implants for Treatment (AMRIT) pharmacies across the country and if so, the details thereof, State-wise; (b) the number of AMRIT outlets proposed to be opened in various States; (c) the objectives of AMRIT outlets; and (d) the number of patients benefited and the amount saved with the help of AMRIT pharmacies along with details thereof? ANSWER THE MINISTER OF STATE IN THE MINISTRY OF HEALTH AND FAMILY WELFARE (SMT. ANUPRIYA PATEL) (a) to (d): HLL Lifecare Ltd, a Government of India CPSU, is setting up Affordable Medicines and Reliable Implants for Treatment (AMRIT) Pharmacies. The States were advised to consider opening AMRIT pharmacies at their institutions using suitable mechanisms. It aims to provide affordable medicines for treatment of cancer, cardiovascular and other diseases, thereby reducing the out of pocket expenses. The list of states in which this scheme has been implemented is at Annexure. As on 15th January 2018, 111 AMRIT Pharmacies are operational in 20 States wherein 52.49 lakh patients have been benefitted and Rs. 486.55 crores worth of drugs sold at Rs. 219.35 crores, resulting in saving of Rs. 267.20 crores for patients. …………. ANNEXURE LIST OF AMRIT DEENDAYAL PHARMACIES -

Normal Pages 8/19/2021 10:34 PM Page 1
Page 1 August 20_Normal Pages 8/19/2021 10:34 PM Page 1 5 Assam CM lays foundation stone of ` N O 3 crore agri ecotourism project at AAU I G E Subungthini Thandwi Bineswar R Brahma remembered in Kokrajhar 7 Calcutta HC orders CBI, SIT probes N O in post-poll violence cases in Bengal I T A Army JCO, terrorist killed in N encounter in J-K’s Rajouri www.thehillstimes.in Voice of the hills people HE ILLS IME` S Vol .No. XXII Issue No. 213 Regd. NGG-709 RNI-ASSENG/2000/128T62 DIPHU, KARBI AHNGLONG, FRI DAY, AUGUST 20, 2021T PAGES 8 PRICE 5 Himanta visits historic Jorhat Central Jail, pays homage to martyr Kushal Konwar AHSEC to continue A reflection to the ALTE ‘in lieu of MIL’ in HS as earlier Journey of 100 days HT Bureau GUWAHATI, Aug 19: Reversing Dr. Himanta Biswa Sarma JoHTr Cohrrespaondtent jail to become heritage site its own order of withdrawing ‘Al - Chief Minister, Assam JORHAT, Aug 19: The historic ternative English’ (ALTE) from the Jorhat Central Jail, where martyr Govt to restructure MIL from the 2021-22 academic iding on the good wishes and blessings of the people of Assam, Kushal Konwar was executed session, the Assam Higher Second - our government assumed the reins of power exactly before 100 would be made into a heritage site functioning of medical ary Education Council (AHSEC) days. We embarked into a journey to bring about a substantive and as it was inextricably linked with has decided to continue ALTE as a meaningful transformation. -
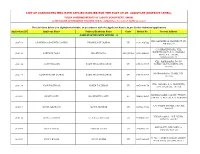
List of Candidates Who Have Applied Earlier for the Post of Jr. Assistant (District Level)
LIST OF CANDIDATES WHO HAVE APPLIED EARLIER FOR THE POST OF JR. ASSISTANT (DISTRICT LEVEL), UNDER COMMISSIONERATE OF LABOUR DEPARTMENT, ASSAM, AS PER EARLIER ADVERTISEMENT PUBLISHED VIDE NO. JANASANYOG/ D/11915/17, DATED 20-12-2017 The List Given below is in Alphabetical Order, in accordance with the Applicant Name ( As per Earlier Submited Application) Application ID Applicant Name Fathers/Husbands Name Caste Mobile No. Present Address NAME STARTING WITH LETTER: - 'A' VILL DAKSHIN MOHANPUR PT VII, 200718 A B MEHBOOB AHMED LASKAR NIYAMUDDIN LASKAR UR 9101308522 PIN 788119 C/O BRAJEN BORA, VILL. KSHETRI GAON, P.O. CHAKALA 206441 AANUPAM BORA BRAJEN BORA OBC/MOBC 9401696850 GHAT, P.S. JAJORI, NAGAON782142 VILL- MAIRAMARA, PO+PS- 200136 AASIF HUSSAIN ZAKIR HUSSAIN LASKAR UR 8753915707 HOWLY, DIST-BARPETA, PIN- 781316 MOIRAMARA PO-HOWLI, PIN- 200137 AASIF HUSSAIN LASKAR ZAKIR HUSSAIN LASKAR UR 8753915707 781316 VILL.-NAPARA, P.O.-HORUPETA, 200138 ABAN TALUKDAR NAREN TALUKDAR UR 9859404178 DIST.-BARPETA, 781318 NARENGI ASEB COLONY, TYPE IV, 203003 ABANI DOLEY KASHINATH DOLEY SC 7664836895 QTR NO. 4, GHY-26, P.O. NARENGI C/O SALMA STORES, GAR ALI, 202015 ABDUL GHAFOOR ABDUL MANNAN UR 8876215529 JORHAT VILL&P.O.&P.S.:- NIZ-DHING, 206442 ABDUL HANNAN LT. ABDUL MOTALIB UR 9706865304 NAGAON-782123 MAYA PATH, BYE LANE 1A, 203004 ABDUL HAYEE FAKHAR UDDIN UR 7002903504 SIXMILE, GHY-22 H. NO. 4, PEER DARGAH, SHARIF, 203005 ABDUL KALAM ABDUL KARIM UR 9435460827 NEAR ASEB, ULUBARI, GHY 7 HATKHOLA BONGALI GAON, CHABUA TATA GATE, LITTLE AGEL 201309 ABDUL KHAN NUR HUSSAIN KHAN UR 9678879562 SCHOOL ROAD, CHABUA, DIST DIBRUGARH 786184 BIRUBARI SHANTI PATH, H NO. -

Orth East Zone, NHRC to Central Jail at Jorhat
Report on the visit of Shri Umesh Kumar, Special Rapporteur, ~orthEast Zone, NHRC to Central Jail at Jorhat I I I 1. Introduction: I I Central Jail, Jorhat (formerly known as District Jail and later rechristened as Central Jail W.B.~ 19'4aY of September. 2002) was constructed during the British rule rr; the year 1909 AD and was opened in the year of 1911 AD. With Central Jall, or hat having been in existence since the freedom struggle, it bears the rest~monyof historical significance and 6 events that shaped the modern day India. Many prominent Freedom Fighters like the grea! Vaisnavite Social Reforn-,e: P~tambarDev Goswami, former President of lndia Fakaruddin Ali Ahrned, former Chief Minister of Assam Bimala Prasad Chaliha, etc were detained in this jail by the British for their role in freedom movement. There'is a Pitambsi Clev Goswami Memor~al Pr~soninside this jail inauglrrated in the year 2012 when this jail completed its Centenary year. Further, Martyr Kushal Konwar was hanged in this jail on 15th June, 1943. Central Jail Jorhat is the only Jail in the entire North-East of lndia where Capital PunishmentlExecut~onof Condemned prisoner is carried Central Jail. Jorhat is meant for Centralized Detention & correctional treatment of offenders sentenced to various length of imprisonment as well as offenders sent to Judicial Custody. Additionally, it - has been notified as Detention Centre for aetention of Declared Foreign Nationals (DFNs) vide Govt. Notification Ordsr No.?LB.121/2015/44 dtd. 24.09.2015. Accordirlgly all Declared Foreign Nationals (DFNs-Female) apprehended in Sadiya/Dibrgarh/Tinsi~kia arid DFNs (both MaleiFernale) apprehended ill the districts cf Si\tasayar/Jorhat/ MajulilKarbi- Anglong/Golaghat/Ci.laraideo and West Karbi-Anglong are being sent to this detention camp for detention and subsequer~ldeportation, if any to their respective countries. -

Captive Elephant Healthcare Programme
Captive Elephant Healthcare Programme Pilot Project Report Ï eco SYSTEMS INDIA Captive Elephant Healthcare Programme Pilot Project Report Sponsored by The Elephant Sanctuary Hohenwald, Tennessee, USA Kushal Konwar Sarma Parag Jyoti Deka Apurba Chakraborty Nandita Hazarika Ï eco SYSTEMS INDIA Wildlife Health Unit THIS PROJECT WAS SUPPORTED BY The Elephant Sanctuary P.O. Box 393, Hohenwald, TN 38462, USA. Phone +1-931-796-6500 Fax +1-931-796-4810 Email [email protected] Website http://www.elephants.com FOR ADDITIONAL INFORMATION CONTACT EcoSystems-India NE Centre, 402 Swati Apts., G. S. Road, Christianbasti Guwahati 781005, Assam, India. Phone +91-361-234 4646 Mobile +91-98640 63637 Email [email protected] or Dr Kushal Konwar Sarma Gajamukta, No.1 Janapath, Khanapara Guwahati 781022, Assam, India. Phone +91-361-222 1682 Mobile +91-98640 63873 Email [email protected] 2003 EcoSystems-India Citation: Sarma K K, Deka P J D, Chakraborty A and Hazarika N. 2003. Captive Elephant Healthcare Programme: Pilot Project Report. Ecosystems-India, Guwahati. Cover photographs: A. Christy Williams Inside photographs: A. Christy Williams and Kushal Konwar Sarma Layout: Goutam Narayan CONTENTS Executive Summary ……………………………………………… ii Project Team ……………………………………………………… iv Acknowledgement ………………………………………………… iv Background ………………………………………………………… 1 Project objective …………………………………………………… 1 Area of operation …………………………………………………… 2 Methodology ………………………………………………………… 2 Camp activities ……………………………………………………… 3 Medical procedures ………………………………………………… -

Bengal, Bihar, Jharkhand, Odisha, Assam, Arunachal Pradesh, Manipur, Meghalaya, Mizoram, Nagaland and Tripura
DICTIONARY OF MARTYRS INDIA’S FREEDOM STRUGGLE (1857-1947) Vol. 4 Bengal, Bihar, Jharkhand, Odisha, Assam, Arunachal Pradesh, Manipur, Meghalaya, Mizoram, Nagaland and Tripura Mangal Pande Jatindra Nath Mukherjee alias Bagha Jatin Photo Courtesy: NCERT ii Dictionary of Martyrs: India’s Freedom Struggle (1857-1947) Vol. 3 DICTIONARY OF MARTYRSMARTYRS INDIA’S FREEDOM STRUGGLE (1857-1947) Vol. 4 Bengal, Bihar, Jharkhand, Odisha, Assam, Arunachal Pradesh, Manipur, Meghalaya, Mizoram, Nagaland and Tripura General Editor Arvind P. Jamkhedkar Chairman, ICHR Executive Editor Rajaneesh Kumar Shukla Member Secretary, ICHR Research Consultant Amit Kumar Gupta Research and Editorial Team Ashfaque Ali Md. Naushad Ali Md. Shakeeb Athar Muhammad Niyas A. Published by MINISTRY OF CULTURE, GOVERNMENT OF IDNIA & INDIAN COUNCIL OF HISTORICAL RESEARCH iv Dictionary of Martyrs: India’s Freedom Struggle (1857-1947) Vol. 3 MINISTRY OF CULTURE, GOVERNMENT OF INDIA and INDIAN COUNCIL OF HISTORICAL RESEARCH First Edition 2016 Published by MINISTRY OF CULTURE Government of India and INDIAN COUNCIL OF HISTORICAL RESEARCH 35, Ferozeshah Road, New Delhi - 110 001 © ICHR & Ministry of Culture, GoI No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. ISBN 978-81-938176-0-5 Printed in India by MANAK PUBLICATIONS PVT. LTD B-7, Saraswati Complex, Subhash Chowk, Laxmi Nagar, New -

Planter-Raj to Swaraj Freedom Struggle and Electoral Politics in Assam 1826-1947
Planter-Raj to Swaraj Freedom Struggle and Electoral Politics in Assam 1826-1947 Amalendu Guha Indian Council of Historical Research 35 Ferozeshah Road New Delhi iioooi . Foreword The Indian Council of Historical Research was presented with a request from the Minister for Education and Social Welfare, which he had received in 1972 from Shri Raj Bahadur, then Union Minis- ter for Parliamentary Affairs, and Shri K.C. Pant, then Union Minister for Home Affairs, for bringing out a series of books on the role of the central and state legislatures during our freedom strug- gle to mark the 25th Anniversary in 1972 of India’s attainment of independence The Council gladly accepted this assignment, and Professor Manoranjan Jha’s work on Role of the Central Legislatures in the Free- dom Struggle and Dr Amit Kumar Gupta’s book on North West Frontier Province Legislature and Freedom Struggle 1932-47 have al- ready been published as a result of its efforts. The third book to come out in this series is the present work on Assam Legislature by Professor Amalendu Guha, Professor of Economic History at the Centre for Studies in Social Sciences, Calcutta. The book has been written in consultation with an Editorial Board for the entire project, under the chairmanship of Professor S. Gopal, but like all other authors in this project Dr Guha has been given complete academic freedom to express views based on his research. Professor Guha has not only presented a detailed account of the evolution of the Provincial Legislature of Assam in the context of general political developments in the Province, but has also pro- vided valuable background for an understanding of the colonial oiii Foreword socio-economic structure. -

For Media Use Only PRESS INFORMATION BUREAU
7th Year of Publication For Media Use Only (A MONTHLY JOURNAL PUBLISHED BY PIB GUWAHATI) VOL. VII ISSUE VIII AUGUST 2005 PRESS INFORMATION BUREAU GOVERNMENT OF INDIA ------------------------------------------------------------------------------------------------------------------------------ TEL : 2540160 ASHRAM ROAD WEBSITE : http://www.pibguwahati.nic.in 2522859 ULUBARI E-MAIL : [email protected] FAX : 2540793 GUWAHATI [email protected] Vol.VII Issue VIII AUGUST 2005 C O N T E N T S IN THIS ISSUE The News Round-Up Independence Day Feature : Martyr Kushal Konwar Feature : Lived as an Emperor, Died as a Saint Feature : Indian Independence Struggle and Assamese Women Backgrounder : Forest Survey of India Feature : Defining Poverty in True Sense Feature : Harnessing North East Economic Potential PM’s Speech Highlights : Prime Minister’s Independence Day Address Feature : National Rural Health Mission Feature : Manas – Paradise on Earth Cover Page : Martyr Kushal Konwar Editor : Smt. R. Sonowal Kouli, I.O. Assistant : Shri N. Chowdhury, Steno(Jr.) -------------------------------------------------------------------------------------------------------------- THE NEWS ROUND-UP B. NARZARY NEW DAVP REGIONAL DIRECTOR Shri Bidyasagar Narzary, Director Public Relations, Press Information Bureau, Guwahati has recently taken over as Regional Director of the Directorate of Advertisement & Visual Publicity, Ministry of Information & Broadcasting, Government of India in Guwahati, on his promotion to the Senior Administrative Grade of the