MS Thesis Draft.Docx
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tortricidae (Lepidoptera) from the Valley of Río Gualaceo, East Cordillera in Ecuador, with Descriptions of New Taxa
Acta zoologica cracoviensia, 49B(1-2): 17-53, Kraków, 30 June, 2006 Tortricidae (Lepidoptera) from the Valley of Río Gualaceo, East Cordillera in Ecuador, with descriptions of new taxa Józef RAZOWSKI and Janusz WOJTUSIAK Received: 10 Dec. 2005 Accepted: 9 Jan. 2006 RAZOWSKI J., WOJTUSIAK J. 2006. Tortricidae (Lepidoptera) in the valley of Río Guala- ceo, East Cordillera in Ecuador, with descriptions of new taxa. Acta zoologica cra- coviensia, 49B(1-2): 17-53. Abstract. Tortricidae collected in RRo Gualaceo Valley with special attention to their ele- vational distribution are listed. Three genera and 34 species are described as new: Henri- cus cerussatus sp.n., Bonagota moronaecola sp.n., Dogolion textrix sp.n., Netechma brunneochra sp.n., Netechma nigricunea sp.n., Netechma triangulum sp.n., Netechma chytrostium sp.n., Netechma paralojana sp. n., Romanaria gen.n., Romanaria spasmaria sp.n., Inape cinnamobrunnea sp.n., Badiaria gen.n., Badiaria plagiostrigata sp.n.. Go- rytvesica cidnozodion sp.n., Gorytvesica chara sp.n., Gorytvesica cerussolinea sp.n., Er- nocornutia gualaceoana sp.n., Ernocornutia limona sp.n., Bidorpidia ceramia sp.n., Moronanita gen.n., Moronanita moronana sp.n., Orthocomotis albimarmorea sp.n., Or- thocomotis marmorobrunnea sp.n., Argyrotaenia cacaoticaria sp.n., Sisurcana pallido- brunnea sp.n., Anacrusis erioheir sp.n., Archipimima undulicostata sp.n., Sparganothina flava sp.n., Paramorbia aureocastanea sp.n., Auratonota chlamydophora sp.n., Aura- tonota aurochra sp.n., Epinotia chloana sp.n., Epinotia tenebrica sp.n., Epinotia illepi- dosa sp.n., Epinotia brunneomarginata sp.n., Laculataria nigroapicata sp.n., Gretchena ochrantennae sp.n. Cnephasia iantha MEYRICK is transferred to Inape, Argyroplae inter- missa (MEYRICK)toEpinotia. -

Phylogeny of Tortricidae (Lepidoptera): a Morphological Approach with Enhanced Whole
Template B v3.0 (beta): Created by J. Nail 06/2015 Phylogeny of Tortricidae (Lepidoptera): A morphological approach with enhanced whole mount staining techniques By TITLE PAGE Christi M. Jaeger AThesis Submitted to the Faculty of Mississippi State University in Partial Fulfillment of the Requirements for the Degree of Master of Science in Agriculture and Life Sciences (Entomology) in the Department of Biochemistry, Molecular Biology, Entomology, & Plant Pathology Mississippi State, Mississippi August 2017 Copyright by COPYRIGHT PAGE Christi M. Jaeger 2017 Phylogeny of Tortricidae (Lepidoptera): A morphological approach with enhanced whole mount staining techniques By APPROVAL PAGE Christi M. Jaeger Approved: ___________________________________ Richard L. Brown (Major Professor) ___________________________________ Gerald T. Baker (Committee Member) ___________________________________ Diana C. Outlaw (Committee Member) ___________________________________ Jerome Goddard (Committee Member) ___________________________________ Kenneth O. Willeford (Graduate Coordinator) ___________________________________ George M. Hopper Dean College of Agriculture and Life Sciences Name: Christi M. Jaeger ABSTRACT Date of Degree: August 11, 2017 Institution: Mississippi State University Major Field: Agriculture and Life Sciences (Entomology) Major Professor: Dr. Richard L. Brown Title of Study: Phylogeny of Tortricidae (Lepidoptera): A morphological approach with enhanced whole mount staining techniques Pages in Study 117 Candidate for Degree of Master of -

Diversidad De Plantas Hospederas De Mariposas Diurnas Y Nocturnas (Lepidoptera)
Universidad Nacional Facultad de Ciencias Exactas y Naturales Escuela de Ciencias Biológicas Práctica Profesional Supervisada Diversidad de plantas hospederas de mariposas diurnas y nocturnas (Lepidoptera) en el Sector San Cristóbal, Área de Conservación Guanacaste, Costa Rica Christian G. Herrera Martínez1 Coordinador: M.Sc Ricardo Jiménez Montealegre Tutor: Lic. Rolando Calderón Fallas Asesor: Ph.D. Daniel H. Janzen II Ciclo 2011 [email protected] Índice de contenidos Introducción…………………………………………………………………………..…………...2 Justificación……………………………………………...………………………………..………5 Objetivos General…………………………………………………………………………..………...5 Específico…………....…………………………………………………………….………5 Descripción de la Institución y Proyecto………………………………………….………….6 Materiales y Métodos…………………………………………………………….………………7 Resultados…………………………………………………..………………………..……………9 Discusión……………………………………..…………………………………………..………16 Conclusiones………………………………………………………………………..……..........20 Referencias bibliográficas……………………………………………………………..……..21 Experiencia adquirida al realizar la PPS……………………………………………………27 1 INTRODUCCIÓN El conocimiento de la biodiversidad requiere considerar los diferentes niveles jerárquicos de organización de los organismos (genes, especies, poblaciones, comunidades y ecosistemas), asimismo, se tiene que tomar en cuenta la composición, estructura y funcionalidad. Por lo tanto, para evaluar la biodiversidad es importante conocer los elementos o entidades que la componen. A su vez, la realización de inventarios facilita conocer la estructura y función de diferentes -

Re-Examining the Rare and the Lost: a Review of Fossil Tortricidae (Lepidoptera)
Zootaxa 4394 (1): 041–060 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2018 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4394.1.2 http://zoobank.org/urn:lsid:zoobank.org:pub:6AEE9169-0FC2-4728-A690-52FFA1707FC0 Re-examining the rare and the lost: a review of fossil Tortricidae (Lepidoptera) MARIA HEIKKILÄ1,6, JOHN W. BROWN2, JOAQUIN BAIXERAS3, WOLFRAM MEY4 & MIKHAIL V. KOZLOV5 1Finnish Museum of Natural History, Zoology Unit, FI-00014 University of Helsinki, Finland. E-mail: [email protected] 2National Museum of Natural History, NHB E-516 MRC 168, Washington, DC 20013-7012, U.S.A. E-mail: [email protected] 3Cavanilles Institute of Biodiversity and Evolutionary Biology, University of Valencia, C/ Catedrático José Beltran, 2, 46980 Valencia, Spain. E-mail: [email protected] 4Museum für Naturkunde, Leibniz-Institut für Evolutions- und Biodiversitätsforschung, Invalidenstrasse, 43, D-10115 Berlin, Ger- many. E-mail: [email protected] 5Section of Ecology, Department of Biology, University of Turku, FI-20014 Turku, Finland. E-mail: [email protected] 6Corresponding author Abstract We re-evaluate eleven fossils that have previously been assigned to the family Tortricidae, describe one additional fossil, and assess whether observable morphological features warrant confident assignment of these specimens to this family. We provide an overview of the age and origin of the fossils and comment on their contribution towards understanding the phy- logeny of the Lepidoptera. Our results show that only one specimen, Antiquatortia histuroides Brown & Baixeras gen. and sp. nov., shows a character considered synapomorphic for the family. -

Lepidoptera: Tortricidae) Collected by B
Acta zoologica cracoviensia, 47(3-4): 249-261, Kraków, 31 December, 2004 Tortricinae and Chlidanotinae (Lepidoptera: Tortricidae) collected by B. LANDRY in Ecuador Józef RAZOWSKI Received: 15 Sep., 2004 Accepted for publication: 1 Oct., 2004 RAZOWSKI J. 2004. Tortricinae and Chlidanotinae (Lepidoptera: Tortricidae) collected by B. LANDRY in Ecuador. Acta zoologica cracoviensia, 47(3-4): 249-261. Abstract. Of thirteen species listed one genus, three Euliini species (Pseudomeritastis em- phanes, Netechmodes landryi, Thalleulia gracilescens), three species of Archipini (Clep- sis parva, C. parassensus, C. assensiodes), two species of Atteriini (Sisurcana leptina, Anacrusis rubida) and one of Chlidanotini (Monortha procera) are described as new. Fe- male of Henricus metalliferus RAZOWSKI &PELZ is described. Key words: Lepidoptera, Tortricidae, new taxa, Ecuador. Józef RAZOWSKI, Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, S³awkowska 17, 31-016 Kraków, Poland E-mail: [email protected] I. INTRODUCTION Recently the Tortricidae of Ecuador are efficiently studied thanks to intense collecting by V.O. BECKER,V.PELZ,J.WOJTUSIAK, and B. LANDRY who chiefly studied the Galapagos Islands. The project of was presented by RAZOWSKI and PELZ (2001) who successively are publishing on the Ec- uadoran material. The present paper is a continuation of this project. This paper is based on the material collected in the Pichincha, Northern Ecuador in which no- body collected tortricines to date (S00o 01.235’ W078o W078o64.600’). All specimens were collected within three days, 8-10 May 2002 in a forest camp at an altitude of about 1300 m above sea level with the use of the ultra violet light by B. -

Lepidoptera: Tortricidae) SHILAP Revista De Lepidopterología, Vol
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Razowski, J. Atteriini collected in Brazil, with descriptions of four new species (Lepidoptera: Tortricidae) SHILAP Revista de Lepidopterología, vol. 32, núm. 128, diciembre, 2004, pp. 347-353 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45512818 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative 347 J. Razowski 3 3/1/77 18:54 Página 347 SHILAP Revta. lepid., 32 (128), 2004: 347-353 SRLPEF ISSN:0300-5267 Atteriini collected in Brazil, with descriptions of four new species (Lepidoptera: Tortricidae) J. Razowski Abstract Some notes on the tribe Atteriini and the genus Sisurcana are provided. Four new species, viz., Sicurcana bra- siliana Razowski, sp. n., Anacrusis russomitrana Razowski & Becker, sp. n., Archipimima consenteana Razowski, sp. n., and A. vermelhana Razowski, sp. n. are described from Brazil. KEY WORDS: Lepidoptera, Tortricidae, Atteriini, new species, Brazil Atteriini recogidos en Brasil, con la descripción de cuatro nuevas especies (Lepidoptera: Tortricidae) Resumen Se presentan algunas notas sobre la tribu Atteriini y el género Sisurcana. Cuatro nuevas especies, véase, Sicur- cana brasiliana Razowski, sp. n., Anacrusis russomitrana Razowski & Becker, sp. n., Archipimima consenteana Ra- zowski, sp. n., y A. vermelhana Razowski, sp. n., se describen de Brasil. PALABRAS CLAVE: Lepidoptera, Tortricidae, Atteriini, nuevas especies, Brasil Introduction In the interpretation by POWELL (1986) Atteriini are treated as a separate tribe comprising five Neotropical genera. -

Tinacrucis Noroesta, New Species, North America's
VOLUME 63, NUMBER 1 27 Journal of the Lepidopterists’ Society 63(1), 2009, 27–30 TINACRUCIS NOROESTA, NEW SPECIES, NORTH AMERICA’S LARGEST TORTRICINE MOTH (TORTRICIDAE: ATTERIINI) JERRY A. POWELL Essig Museum of Entomology, University of California, Berkeley, 94720, e-mail: [email protected] ABSTRACT. The Neotropical tribe Atteriini is briefly characterized, and Tinacrucis noroesta is newly described in order to make the name available for a forthcoming book on moths of western North America. This species, the northernmost of the tribe, occurs in the Sierra Madre Occidental, Mexico, and mountains of southern Arizona, where it was first collected in 1927. The larvae are believed to be polyphagous leaf rollers on broadleaf trees and shrubs but have not been discovered in the field. Additional Key words: Egg mass adornment scaling, sexual dimorphism, Chihuahua, Durango, Arizona, leaf-roller. The Atterini comprises one of the smallest and most Tinacrucis Powell, 1986, is distinguished by having well defined tribes of the Tortricinae. Its monophyly is elaborate antennal setulae in males, the costa sinuate in convincingly supported by a suite of uniquely derived both sexes (unmodified in male Anacrucis and morphological and behavioral characters associated with Templemania), markedly different size and FW pattern oviposition (Powell 1986). The adults are among the between the sexes (though not as extreme as in Tina), largest tortricid moths in the Western Hemisphere, FW greatly enlarged A8 tergum, forming a hood enclosing length ranging to 22 mm. Females are conspicuously the male genitalia. Tinacrucis includes six named larger than males, and there is pronounced sexual species and several undescribed in Mexico and Central dimorphism in wing shape and color patterns in some America. -

Redalyc.Systematic and Faunistic Data on Neotropical Tortricidae
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España Razowski, J.; Becker, V. O. Systematic and faunistic data on Neotropical Tortricidae: Phricanthini, Tortricini, Atteriini, Polyorthini, Chlidanotini (Lepidoptera: Tortricidae) SHILAP Revista de Lepidopterología, vol. 39, núm. 154, junio, 2011, pp. 161-181 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45521389004 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative 161-181 Systematic and faunisti 10/6/11 11:26 Página 161 SHILAP Revta. lepid., 39 (154), junio 2011: 161-181 CODEN: SRLPEF ISSN:0300-5267 Systematic and faunistic data on Neotropical Tortricidae: Phricanthini, Tortricini, Atteriini, Polyorthini, Chlidanotini (Lepidoptera: Tortricidae) J. Razowski & V. O. Becker Abstract 30 species of the four above mentioned tribes of Tortricidae are recorded and discussed. The following 13 species are described as new; Atteriini: Holoptygma braulio Razowski & Becker, sp. n., Anacrusis epidicta Razowski & Becker, sp. n., A. subruptimacula Razowski & Becker, sp. n., A. turrialbae Razowski & Becker, sp. n., Sisurcana itatiaiae Razowski & Becker, sp. n., S. valida Razowski & Becker, sp. n., S. atricaput Razowski & Becker, -

Download Download
July 26 2019 INSECTA 12 urn:lsid:zoobank. A Journal of World Insect Systematics org:pub:677567AB-85B6-4BAD- UNDI M 92FF-2336C714E4F9 0720 New larval host records for Tortricidae (Lepidoptera) from an Ecuadorian Andean cloud forest John W. Brown Department of Entomology National Museum of Natural History Washington, DC, USA Lee A. Dyer Department of Biology University of Nevada Reno, NV, USA Santiago Villamarín-Cortez Instituto Nacional de Biodiversidad del Ecuador Quito, Ecuador Danielle Salcido Department of Biology University of Nevada Reno, NV, USA Date of issue: July 26, 2019 CENTER FOR SYSTEMATIC ENTOMOLOGY, INC., Gainesville, FL John W. Brown, Lee A. Dyer, Santiago Villamarín-Cortez and Danielle Salcido New larval host records for Tortricidae (Lepidoptera) from an Ecuadorian Andean cloud forest Insecta Mundi 0720: 1–12 ZooBank Registered: urn:lsid:zoobank.org:pub:677567AB-85B6-4BAD-92FF-2336C714E4F9 Published in 2019 by Center for Systematic Entomology, Inc. P.O. Box 141874 Gainesville, FL 32614-1874 USA http://centerforsystematicentomology.org/ Insecta Mundi is a journal primarily devoted to insect systematics, but articles can be published on any non- marine arthropod. Topics considered for publication include systematics, taxonomy, nomenclature, checklists, faunal works, and natural history. Insecta Mundi will not consider works in the applied sciences (i.e. medical entomology, pest control research, etc.), and no longer publishes book reviews or editorials. Insecta Mundi publishes original research or discoveries in an inexpensive and timely manner, distributing them free via open access on the internet on the date of publication. Insecta Mundi is referenced or abstracted by several sources, including the Zoological Record and CAB Abstracts. -

A-Razowski.Vp:Corelventura
Acta zoologica cracoviensia, 59(2) 2016 ISSN 2299-6060, e-ISSN 2300-0163 Kraków, 30 December, 2016 doi:10.3409/azc.59_2.89 Ó Institute of Systematics and Evolution of Animals, PAS Diagnoses and remarks on the genera of Tortricidae (Lepidoptera). Part 4. Cnephasiini, Ceracini, Atteriini, Sparganothini and Euliini Józef RAZOWSKI Received: 04 October 2016. Accepted: 22 November 2016. Available online: 02 December 2016. RAZOWSKI J. 2016. Diagnoses and remarks on the genera of Tortricidae (Lepidoptera). Part 4. Cnephasiini, Ceracini, Atteriini, Sparganothini and Euliini. Acta zool. cracov.,59(2): 89-151. Abstract. Diagnoses, redescriptions, and remarks are presented on the genera that com- prised the five tortricid tribes Cnephasiini (19 genera), Ceracini (4 genera), Atteriini (9 genera), Sparganothini (20 genera), and Euliini (173 genera). Original references, type species, type localities, synonyms, and zoogeographic regions are provided. Moronata is a synonym of Pseudapina. Raisapoana, omitted in Archipini is added in the appendix. Key words: Lepidoptera, Tortricidae, genera, diagnoses, descriptions * Józef RAZOWSKI, Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, S³awkowska 17, 31-016 Kraków, Poland. E-mail: [email protected] I. INTRODUCTION The number of genera of Tortricidae has increased dramatically over last 40 years; by 2007 there were over 1630 described genera, including synonyms. Many of the older de- scriptions are scattered throughout the literature, and because there are few larger synthetic treatments of the tortricids for most major biogeographic regions, this large number of taxa complicates considerably the work of taxonomists on the faunas of poorly known regions of the planet. In addition, characters that define many of the genera are not clearly articu- lated. -

Razowski J., Wojtusiak J
Genus Vol. 21(4): 585-603 Wrocław, 27 XII 2010 Some Tortricidae from the East Cordillera in Ecuador reared from larvae in Yanayacu Biological Station in Ecuador (Insecta: Lepidoptera) JÓZEF RAZOWSKI1 & JANUSZ WOJTUSIAK2 1Institute of Systematics and Evolution of Animals PAS, Sławkowska 17, 31-016 Kraków, Poland, e-mail: [email protected] 2Zoological Museum, Jagiellonian University, Ingardena 6, Kraków, Poland, e-mail: [email protected] ABSTRACT. Eight species are described from Ecuador (Napo Prov.) as new on the basis of specimens reared from their larvae. These are: Xoser astonyx n. sp., Orthocomotis parandina n. sp., Anacrusis yanayacana n. sp., Anacrusis guttula n. sp., Sisurcana sanguinoventer n. sp., Sisurcana cirrhochroma n. sp., Sparganothina hermosa n. sp., Lypothora roseochraon n. sp. Key words: entomology, taxonomy, Lepidoptera, Tortricidae, Ecuador, new taxa, Andes INTRodUCTIoN The Tortricidae fauna (Lepidoptera) of Ecuador is relatively well studied when compared with other andean countries. The results of the research conducted in the past show that the East Cordillera of Ecuador harbors the highest number of species. Until now, all the research material of neotropical Tortricidae used for studies was obtained in the field by the method of collecting adult moths that have been attracted to the light. No specimens were obtained by rearing them from larvae, or pupae. This paper is the first that is basing on specimens reared from their caterpillars. It adds more data to the overall knowledge of the Tortricidae of Ecuador supplement- ing faunistic lists of species knowing to occur in the East Cordillera (RAZOWSKI & WOJTUSIAK 2006, 2009). The specimens were bred in Yanayacu Research Station in Ecuador as a result of the research program aimed to study larval stages of Neotropical Lepidoptera. -
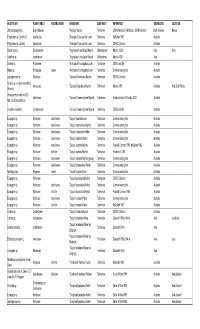
Sorted by Moth Species
HOST PLANT PLANT FAMILY FEEDING NICHE HERBIVORE SUBFAMILY REFERENCE GEOREGION LOCATION Jatropha gossypifolia Euphorbiaceae "Amorbia" depicta Tortricinae CSIRO Mexican Field Station; USNM collection North America Meixco Polystichum sp. ("prolifera") Aspidiaceae "Anisogona" placoxantha Lower Tortricinae McQuillan 1992 Australia Polystichum sp. (as fern) Aspidiaceae "Anisogona" placoxantha Lower Tortricinae CSIRO Collection Australia Cordia myxa L. Boraginaceae "Argyroploce" cenchropis Meyrick Olethreutinae Meyrick 1920a Asia India Acanthus sp. Acanthaceae "Argyroploce" vinculigera Meyrick Olethreutinae Meyrick 1939 Asia Banksia sp. Proteaceae "Arotrophora" cosmoplaca Lower Tortricinae CSIRO card file Australia Hakea sp. Proteaceae leaves "Arotrophora" cosmoplaca Lower Tortricinae Common rearing files Australia Leptospermum sp. Myrtaceae "Cacoecia" desmotana Meyrick Tortricinae CSIRO Collection Australia Senecio sp. (as plant resembling Asteraceae "Cacoecia" jugicolana Meyrick Tortricinae Meyrick 1881 Australia New South Wales Senecio) Osteospermum ecklonis (DC.) Asteraceae "Cacoecia" mnemosynana Meyrick Tortricinae Herbison-Evans & Crossley 2003 Australia Norl. (as Dimorphotheca) Goodenia ovata Sm. Goodeniaceae "Cacoecia" mnemosynana Meyrick Tortricinae CSIRO card file Australia Eucalyptus sp. Myrtaceae dead leaves "Capua" ceramica Lower Tortricinae Common rearing files Australia Eucalyptus sp. Myrtaceae dead leaves "Capua" cnaphalodes Meyrick Tortricinae Common rearing files Australia Eucalyptus sp. Myrtaceae dead leaves "Capua" constrictana