Phosphorus Status in Soils of Eastern Dry Zone, Karnataka, India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

HŒ臬 A„簧綟糜恥sµ, Vw笑n® 22.12.2019 Š U拳 W
||Om Shri Manjunathaya Namah || Shri Kshethra Dhamasthala Rural Development Project B.C. Trust ® Head Office Dharmasthala HŒ¯å A„®ãtÁS®¢Sµ, vw¯ºN® 22.12.2019 Š®0u®± w®lµu® îµ±°ªæX¯Š®N®/ N®Zµ°‹ š®œ¯‡®±N®/w®S®u®± š®œ¯‡®±N® œ®±uµÛ‡®± wµ°Š® wµ°î®±N¯r‡®± ªRq® y®‹°£µ‡®± y®ªq¯ºý® D Nµ¡®w®ºruµ. Cu®Š®ªå 50 î®±q®±Ù 50 Oʺq® œµX®±Ï AºN® y®lµu®î®Š®w®±Ý (¬šµ¶g¬w®ªå r¢›Š®±î®ºqµ N®Zµ°‹/w®S®u®± š®œ¯‡®±N® œ®±uµÛSµ N®xÇ®Õ ïu¯ãœ®Áqµ y®u®ï î®±q®±Ù ®±š®±é 01.12.2019 NµÊ Aw®æ‡®±î¯S®±î®ºqµ 25 î®Ç®Á ï±°Š®u®ºqµ î®±q®±Ù îµ±ªæX¯Š®N® œ®±uµÛSµ N®xÇ®Õ Hš¬.Hš¬.HŒ¬.› /z.‡®±±.› ïu¯ãœ®Áqµ‡µ²ºvSµ 3 î®Ç®Áu® Nµ©š®u® Aw®±„Â®î® î®±q®±Ù ®±š®±é 01.12.2019 NµÊ Aw®æ‡®±î¯S®±î®ºqµ 30 î®Ç®Á ï±°Š®u®ºqµ ) î®±±ºvw® œ®ºq®u® š®ºu®ý®Áw®NµÊ B‡µ±Ê ¯l®Œ¯S®±î®¼u®±. š®ºu®ý®Áw®u® š®Ú¡® î®±q®±Ù vw¯ºN®î®w®±Ý y®äqµã°N®î¯T Hš¬.Hº.Hš¬ î®±²©N® ¯Ÿr x°l®Œ¯S®±î®¼u®±. œ¯cŠ¯u® HŒ¯å A„®ãtÁS®¢Sµ A†Ãw®ºu®wµS®¡®±. Written test Sl No Name Address Taluk District mark Exam Centre out off 100 11 th ward near police station 1 A Ashwini Hospete Bellary 33 Bellary kampli 2 Abbana Durugappa Nanyapura HB hally Bellary 53 Bellary 'Sri Devi Krupa ' B.S.N.L 2nd 3 Abha Shrutee stage, Near RTO, Satyamangala, Hassan Hassan 42 Hassan Hassan. -

Chintamani Assembly Karnataka Factbook
Editor & Director Dr. R.K. Thukral Research Editor Dr. Shafeeq Rahman Compiled, Researched and Published by Datanet India Pvt. Ltd. D-100, 1st Floor, Okhla Industrial Area, Phase-I, New Delhi- 110020. Ph.: 91-11- 43580781-84 Email : [email protected] Website : http://www.indiastatelections.com Online Book Store : www.indiastatpublications.com Report No. : AFB/KA-143-0121 ISBN : 978-93-87130-20-3 First Edition : January, 2018 Third Updated Edition : January, 2021 Price : Rs. 11500/- US$ 310/- © Datanet India Pvt. Ltd. All rights reserved. No part of this book may be reproduced, stored in a retrieval system or transmitted in any form or by any means, mechanical photocopying, photographing, scanning, recording or otherwise without the prior written permission of the publisher. Please refer to Disclaimer at page no. 209 for the use of this publication. Printed in India Contents No. Particulars Page No. Introduction 1 Assembly Constituency - (Vidhan Sabha) at a Glance | Features of Assembly 1-2 as per Delimitation Commission of India (2008) Location and Political Maps Location Map | Boundaries of Assembly Constituency - (Vidhan Sabha) in 2 District | Boundaries of Assembly Constituency under Parliamentary 3-10 Constituency - (Lok Sabha) | Town & Village-wise Winner Parties- 2019, 2018, 2014, 2013 and 2009 Administrative Setup 3 District | Sub-district | Towns | Villages | Inhabited Villages | Uninhabited 11-25 Villages | Village Panchayat | Intermediate Panchayat Demographics 4 Population | Households | Rural/Urban Population -

Of 426 AUTO YEAR IVPR SRL PAGE DOB NAME ADDRESS STATE PIN
Page 1 of 426 AUTO YEAR IVPR_SRL PAGE DOB NAME ADDRESS STATE PIN REG_NUM QUALIF MOBILE EMAIL 7356 1994S 2091 345 28.04.49 KRISHNAMSETY D-12, IVRI, QTRS, HEBBAL, KARNATAKA VCI/85/94 B.V.Sc./APAU/ PRABHODAS BANGALORE-580024 KARNATAKA 8992 1994S 3750 425 03.01.43 SATYA NARAYAN SAHA IVRI PO HA FARM BANGALORE- KARNATAKA VCI/92/94 B.V.Sc. & 24 KARNATAKA A.H./CU/66 6466 1994S 1188 295 DINTARAN PAL ANIMAL NUTRITION DIV NIANP KARNATAKA 560030 WB/2150/91 BVSc & 9480613205 [email protected] ADUGODI HOSUR ROAD AH/BCKVV/91 BANGALORE 560030 KARNATAKA 7200 1994S 1931 337 KAJAL SANKAR ROY SCIENTIST (SS) NIANP KARNATAKA 560030 WB/2254/93 BVSc&AH/BCKVV/93 9448974024 [email protected] ADNGODI BANGLORE 560030 m KARNATAKA 12229 1995 2593 488 26.08.39 KRISHNAMURTHY.R,S/ #1645, 19TH CROSS 7TH KARNATAKA APSVC/205/94,VCI/61 BVSC/UNI OF 080 25721645 krishnamurthy.rayakot O VEERASWAMY SECTOR, 3RD MAIN HSR 7/95 MADRAS/62 09480258795 [email protected] NAIDU LAYOUT, BANGALORE-560 102. 14837 1995 5242 626 SADASHIV M. MUDLAJE FARMS BALNAD KARNATAKA KAESVC/805/ BVSC/UAS VILLAGE UJRRHADE PUTTUR BANGALORE/69 DA KA KARANATAKA 11694 1995 2049 460 29/04/69 JAMBAGI ADIGANGA EXTENSION AREA KARNATAKA 591220 KARNATAKA/2417/ BVSC&AH 9448187670 shekharjambagi@gmai RAJASHEKHAR A/P. HARUGERI BELGAUM l.com BALAKRISHNA 591220 KARANATAKA 10289 1995 624 386 BASAVARAJA REDDY HUKKERI, BELGAUM DISTT. KARNATAKA KARSUL/437/ B.V.SC./GAS 9241059098 A.I. KARANATAKA BANGALORE/73 14212 1995 4605 592 25/07/68 RAJASHEKAR D PATIL, AMALZARI PO, BILIGI TQ, KARNATAKA KARSV/2824/ B.V.SC/UAS S/O DONKANAGOUDA BIJAPUR DT. -

In the High Court of Karnataka at Bangalore
1 IN THE HIGH COURT OF KARNATAKA AT BANGALORE DATED THIS THE 09 TH DAY OF JULY, 2012 PRESENT THE HON’BLE MR. JUSTICE N.KUMAR AND THE HON’BLE MR.JUSTICE H.S.KEMPANNA W.P. NOS.48210-48253/2011& 48255-48531/2011 (S-KAT) C/W W.P. NOS.149-330/2012 (S-KAT) W.P.NOS.851-894/2012 (S-KAT) W.P. NO.1075/2012 (S-KAT) W.P. NOS.1207 & 1208/2012 (S-KAT) W.P. NOS.1259-1287/2012 (S-KAT) W.P. NOS.1339-1409/2012 (S-KAT) W.P. NOS.1707-1734/2012 (S-KAT) W.P. NOS.2476-2517/2012 (S-KAT) W.P. NOS.2630-2643/2012 (S-KAT) W.P. NOS.3202-3203/2012 (S-KAT) W.P. NOS.4347-4350/2012 (S-KAT) W.P. NOS.9296-9301/2012 (S-KAT) W.P. NO.3110/2012 (S-KAT) W.P. NOS.15577-15578/2012 (S-KAT) W.P. NOS.15368-370, 15373-375 & 15378-390/2012 (S- KAT) W.P. NOS.15188-15196 & 15198 /2012 (S-KAT) W.P. NOS.11780-783/2012 (S-KAT) W.P. NO.11779/2012 (S-KAT) W.P. NOS.21111-21116/2012 (S-KAT) W.P. NOS.48210-48253/2011 & 48255-48531/2011 BETWEEN: 1) SRI. H.MALLIKARJUNAIAH, S/O. HONGAIAH, AGED ABOUT 39 YEARS, CLUSTER RESOURCE PERSON, CLUSTER RESOURCE CENTRE, MALAVALLI TOWN, MANDYA DISTRICT 2 2) SRI. K.N. KUMAR, S/O. NANJEGOWDA, AGED ABOUT 43 YEARS, CLUSTER RESOURCE PERSON, CLUSTER RESOURCE CENTRE, MUTTEGERE, MANDYA NORTH, MANDYA DISTRICT 3) SRI. BASAVARAJU .P, S/O. -

Government of Karnataka Revenue Village, Habitation Wise Aided and Unaided Neighbourhood Schools
Government of Karnataka O/o Commissioner for Public Instruction, Nrupatunga Road, Bangalore - 560001 Revenue village, Habitation wise Aided and UnAided Neighbourhood Schools - 2015 RURAL Habitation Name School Code Management Lowest High Entry type class class class Habitation code / Ward code School Name Medium Sl.No. District : Chikballapur Block : BAGEPALLY Revenue Village : 29290103017 Pvt Unaided 1 5 Class 1 SARVODAYA PRIMARY SCHOOL 05 - Kannada 1 Revenue Village : BILLUR 29290102001 29290102010 Pvt Unaided 1 5 Class 1 BILLURU SRI LAKSHMI VIDYALAYA BILLUR 05 - Kannada 2 Revenue Village : CHAKAVELU 29290103001 29290103011 Pvt Unaided 1 7 Class 1 CHAKAVELU PSS CHAKAVELU 05 - Kannada 3 29290103001 29290103012 Pvt Unaided 1 7 Class 1 CHAKAVELU MARUTHI HPS CHAKAVELU 05 - Kannada 4 29290103001 29290103014 Pvt Unaided 1 10 Class 1 CHAKAVELU MADEMMA HS 19 - English 5 Revenue Village : CHELUR 29290103401 29290103403 Pvt Aided 1 7 Class 1 CHELURU BHARATHI HPS 05 - Kannada 6 29290103401 29290103424 Pvt Aided 1 7 Class 1 CHELURU KISHORE HPS CHELUR 05 - Kannada 7 29290103401 29290103422 Pvt Unaided 1 7 Class 1 CHELURU ARAVINDA HPS CHELURU 05 - Kannada 8 29290103401 29290103422 Pvt Unaided 1 7 Class 1 CHELURU ARAVINDA HPS CHELURU 19 - English 9 29290103401 29290103423 Pvt Unaided 1 8 LKG CHELURU PRASHANTHI HPS CHELUR ENGLISH 05 - Kannada 10 29290103401 29290103423 Pvt Unaided 1 8 Class 1 CHELURU PRASHANTHI HPS CHELUR ENGLISH 98 - None 11 Revenue Village : G MADDEPALLI 29290105701 29290105711 Pvt Unaided 1 5 Class 1 G MADDEPALLI MADANAGOKULA PRIMARY SCHOOL 05 - Kannada 12 Revenue Village : GOWNIPALLI 29290106101 29290124301 Pvt Unaided 1 5 LKG GOWNIPALLI CHRIST SCHOOL 05 - Kannada 13 e-Governance, CPI office, Bangalore 1/2/2015 -2:54:23 PM 1 Government of Karnataka O/o Commissioner for Public Instruction, Nrupatunga Road, Bangalore - 560001 Revenue village, Habitation wise Aided and UnAided Neighbourhood Schools - 2015 RURAL Habitation Name School Code Management Lowest High Entry type class class class Habitation code / Ward code School Name Medium Sl.No. -

Empanelled Hospital List As on 02-09-2021
Empanelled Hospital list As on 05-10-2021 Helpline Number (Toll Free) : 1800 425 8330 Arogya Mitra Help Line (Toll Free) : 1800 425 2646 Hospital Name District Taluk Division Type Address Phone No Mail-ID Scheme Speciality Trinity Hospital And Bengaluru Bengaluru Bangalore Private Trinity Hospital And 41503434 trinityhospit Ayushman Bharat - CARDIOLOGY,CARDIOTHOR Heart Foundation South Heart Foundation No 27 al@hotmail. Arogya ACIC SURGERY,COVID Sri Rama Mandir Road com Karnataka,Jyothi Bangalore South 560004 Sanjeevini Narayana Hrudayalaya Bengaluru ANEKAL Bangalore Private Narayana Hrudayalaya 9980528300 samconhblr Ayushman Bharat - GENERAL Private Limited Private Limited 258 A @gmail.co Arogya SURGERY,OBSTETRICS AND Bommasandra Industrial m Karnataka,Jyothi GYNAECOLOGY,CARDIOLO Area Anekal Taluk Sanjeevini,Organ GY,CARDIOTHORACIC Bangalore 560099 Transplantion SURGERY,CARDIOVASCULA R SURGERY,ENT,MEDICAL ONCOLOGY,NEONATAL AND PAEDIATRICS,NEUROSURGE RY,ORTHOPAEDICS,RADIAT ION ONCOLOGY,SURGICAL ONCOLOGY,UROLOGY,HEA RT TRANSPLANT,LIVER TRANSPLANT,KIDNEY TRANSPLANT,COVID Samrudhi Eye Hospital Bengaluru BANGALO Bangalore Private Samrudhi Eye Hospital 8042075944 samruddhie Ayushman Bharat - OPHTHALMOLOGY RE No 2 Near Fortuna Vista yehospital@ Arogya Karnataka Apt Kodigehalli Main gmail.com Road Thindlu Vidyaranyapura Post Bangalore West 560097 Page 1 Empanelled Hospital list As on 05-10-2021 Helpline Number (Toll Free) : 1800 425 8330 Arogya Mitra Help Line (Toll Free) : 1800 425 2646 Hospital Name District Taluk Division Type Address Phone -

Government AYUSHMAN BHARAT
AYUSHMAN BHARAT - AROGYA KARNATAKA EMPANELLED HOSPITALS LIST Govt/Priv Sl.no Hospital Name Address District Taluk Division Contact Mail id Scheme Speciality ate Government Community Health Centre Obstetrics and VijayapuraDevanahalli Ayushman gynaecology Community Health Centre Road Vijayapura Bangalore chcvijayapura@g 1 Bangalore Devanahalli govt 8027668505 Bharat - Arogya Dental Vijayapura Devanhalli division mail.com Karnataka Simple secondary general TalukBengaluru Rural- procedure 562135 Obstetrics and Ayushman Community Health Centre B M Road Kengeri Kote Bangalore girijagowdab@g gynaecology Paediatrics 2 Bangalore Bengaluru govt 8028483265 Bharat - Arogya Kengeri Bangalore 560060 division mail.com Simple secondary General Karnataka procedure Paediatric surgeries Community Health Centre Obstetrics and Ayushman Community Health Centre ThyamagondluNear Police Bangalore thyamagondluchc gynaecology 3 Bangalore Nelemangala govt 8027731202 Bharat - Arogya Thyamagondlu StationBangalore - Rural- division @gmail.com Dental Karnataka 562132 Simple secondary General procedure Paediatric Surgery Community Health Center Ayushman General Medicine Community Health Center Near Water Bangalore mophcavalhalli@ 4 Bangalore Bengaluru govt 8028473108 Bharat - Arogya Dental Avalahalli PlantationBangalore - division gmail.com Karnataka Obstetrics and Urban-560049 gynaecology Dental Obstetrics and Ayushman Tavarekere Hobli South Bangalore dr.candrappacercl gynaecology 5 CHC Chandrappa Cercle Bangalore Bengaluru govt 8028438330 Bharat - Arogya TalukBengaluru -

Government of Karnataka Revenue Village, Habitation Wise
Government of Karnataka O/o Commissioner for Public Instruction, Nrupatunga Road, Bangalore - 560001 RURAL Revenue village, Habitation wise Neighbourhood Schools - 2015 Habitation Name School Code Management Lowest Highest Entry type class class class Habitation code / Ward code School Name Medium Sl.No. District : Chikballapur Block : BAGEPALLY Revenue Village : 29290103017 Pvt Unaided 1 5 Class 1 SARVODAYA PRIMARY SCHOOL 05 - Kannada 1 Revenue Village : ACHANGANAPALLI 29290100201 29290100201 Govt. 1 8 Class 1 ACHAGANAPALLI GHPS ACHAGANAPALLI 05 - Kannada 2 29290100202 29290100202 Govt. 1 5 Class 1 BUDIDIGADDAPALLI GLPS BOODIDIGANDDAPALLI 05 - Kannada 3 29290100205 29290100206 Govt. 1 5 Class 1 Y.MARAPPAGARIPALLI GLPS Y.MARAPPAGARAPALLI 05 - Kannada 4 29290100206 29290100205 Govt. 1 5 Class 1 BOMMASANDRA GLPS BOMMASANDRA 05 - Kannada 5 Revenue Village : ACHEPALLI 29290100301 29290100301 Govt. 1 8 Class 1 ACHEPALLI GHPS ACHEPALLI 05 - Kannada 6 Revenue Village : AGATAMADIKE 29290100501 29290100501 Govt. 1 5 Class 1 AGATAMADAKA GLPS AGATAMADIKE 05 - Kannada 7 Revenue Village : ARIGEVARIGUTTA 29290100702 29290100702 Govt. 1 5 Class 1 MAMIDIMAKALAPALLI GLPS MAMIDIMAKALAPALLI 05 - Kannada 8 Revenue Village : BAGEPALLI (RURAL) 29290100902 29290100902 Govt. 1 7 Class 1 KOTHAPALLI GLPS KOTHAPALLI 05 - Kannada 9 Revenue Village : BALAREDDYPALLI 29290101101 29290101101 Govt. 1 5 Class 1 BALAREDDYPALLI GLPS BALAREDDIPALLI 05 - Kannada 10 29290101101 29290101103 Govt. 1 5 Class 1 BALAREDDYPALLI GLPS KAMMARAVARAPALLI 05 - Kannada 11 29290101103 29290101104 -

TL&SS Division,Chintamani
TL&SS Division,Chintamani R & M Works under 74.110-Repair to Plant & Machinery to be taken up FY 2020-21 Rs. in Lakhs Name of SL No Nomenclature Amount Station/Division Apr-20 Estimate for replacement of failed B Phase CT of 8MVA 66/11KV Power Transformer-1 at 66/11KV MUSS 1 66/11KV MUSS Nandhiganahalli 0.06 Nandhiganahalli 66/11KV MUSS Estimate for replacing failed 3X240sqmm XLPE outdoor pothead fixed to 11KV F4-S 2 0.08 Sadali venkatapura feeder at 66/11KV MUSS Sadali Estimate for regularization the tentative work order issued for the work of replacement of faulty Y phase 66KV CT of 100MVA Power Transformer-2 at 220KV RS Chintamani in 3 220KV RS Chintamani 0.13 TL&SSD Chintamani Total 0.27 May-20 66/11KV MUSS Replacement of defective station yard light at 66/11KV MUSS Somanathapura in TL&SSD 4 0.31 Somanathapura Chintamani Providing 11kV class 1x1000sqmm XLPE UG cable to 2x20MVA power transformer for 5 66/11kV Bagepalli 0.86 double run purpose(6run) at 66/11kV Bagepalli 66/11KV MUSS Replacement of defective station yard light at 66/11KV MUSS bagepalli in TL&SSD 6 0.29 bagepalli Chintamani 66/11KV MUSS 7 Supply & Fixing of 1X1000sqmm XLPE H/S outdoor pothead of 'B' phase of 20MVA 0.07 Chintamani Power Transformer-2 at 66/11KV MUSS Chintamani in TL&SSD Chintamani 66/11KV MUSS 8 Painting of 66KV outdoor structure ,66KV brekers ,66KV CT'S 66KV PT'S ,66KV LA'S 0.91 bagepalli power transformer 1 RTCC panel,11KV switch gear panel ,11KV DP structure ,ACPB &DCDB at 66/11KV MUSS bagepalli 66/11KV MUSS Replacemnt of failed station control room -

Chikkaballapura NA I Distillery 1112 L R(17 Cat) 60 5468 C1 Y 31/12/2015 Y 31/12/2015 Y 30/06/2014 N NA N NA N N NA NO NA
F-Register as on 31-03-2019 Capital Registration Type of Applic Applica Registration Num Year of Name of the Size Date of Investme Present Under Bedded organisation/Activity Category No.(XGN Color Numbe ability Water Act bility A HWM BMW Under BATTERY E Waste MSW MSW ber CBMWTF to whom the Remark SI No. PCB ID Identificatio Name of the industry Address of the organization Area/Place/Ward no. Taluk District industrial area/ Product as per Board Classification L/M/S/Micr Establishment nt working Air Act Validity HWM Y/N) BMW Validity PLASTIC E-Waste (Y/N) /Non *(I/M/LB/HC/H/L/C Category Code) R/O/G/W r W Act (Validity) Act Validity (Y/N) PLASTIC (Y/N) (Validity) (Y/N) (Validity) of BMW is handed over s n estate o DD/MM/YY (Lakhs Status RULES bedded E/C/O) (Y/N) (Y/N) RULES (Y/N) Beds of Rs.) Validity 1 11072 1980-81 Hindustan Distilleries (Karnataka) Post Box No.3 B.H.Road, Gowribidanur 1/11980 Ltd., (Formarly Gauri Industries) Tq, Chickaballapur Dist. Gowribidanur Gowribidanur Chikkaballapura NA I Distillery 1112 L R(17 cat) 60 5468 C1 Y 31/12/2015 Y 31/12/2015 Y 30/06/2014 N NA N NA N N NA NO NA 2 12362 1983-84 Sy. No. 36, Kannampalli (V), Bangalore 1/1/1983 KOMUL - Chilling Centre, Road, Chintamani Tq, Chickaballapur Dist. Chintamani Chinthamani Chikkaballapura NA I Milk Chilling 1328 S O 7 297 O Y 30/09/2022 Y 30/09/2022 Y 30/09/2014 N NA N NA N N NA NO NA 3 12358 1988-89 1/6/1988 KOMUL-Sadali Chilling Centre, Sadli, Shidalghatta Tq, Chickaballapur Dist, Sadli Shidlaghatta Chikkaballapura NA I Milk Chilling 1328 S O 7 291 O Y 30/09/2022 Y 30/09/2022 Y 30/09/2014 N NA N NA N N NA NO NA 4 1988-89 Krishnapura, Chikkaballapura (T) & (D) 26/08/1988 Chaitra Chemicals, Krishnapura Chikkaballapura Chikkaballapura NA I Phenyl Products 1344 S O 44 8.3 C1 Y CLOSED Y CLOSED N NA N NA N NA N N NA NO NA Plot No:42(B), KIADB Indl Area, Welding 5 1989-90 13/11/1989 Nucol Weld Pvt Ltd., Chickaballapur Tq & Dist. -

CHIKKABALLAPURA.Pdf
ಕ ೋ풿蓍 ಲಕಾಕರಣ ಕ ೋᲂ飍ರಗಳು (COVID VACCINATION CENTRES) Sl No District CVC Name Category 1 Chikkballapur Alakapura PHC Government 2 Chikkballapur Allipura PHC Government 3 Chikkballapur Ananya Speciality Hospital Private 4 Chikkballapur Bachareddyhalli Government 5 Chikkballapur Bagepalli TH Government 6 Chikkballapur Bashettihalli PHC Government 7 Chikkballapur Batlahalli CHC Government 8 Chikkballapur Beechaganahalli PHC Government 9 Chikkballapur Billur PHC Government 10 Chikkballapur Bodaguru Government 11 Chikkballapur Burudugunte PHC Government 12 Chikkballapur Chakavelu PHC Government 13 Chikkballapur Cheemangala Government 14 Chikkballapur Cheluru PHC Government 15 Chikkballapur Chikkaballapur DH Government 16 Chikkballapur Chikkaballapur UPHC Government 17 Chikkballapur Chilakalanerpu Government 18 Chikkballapur Chinnasandra PHC Government 19 Chikkballapur Chintamani Taluk Hospital Government 20 Chikkballapur Chintamani UHC Government 21 Chikkballapur Cholashettihalli Government 22 Chikkballapur Dapparthi Government 23 Chikkballapur Darinayakanapalya PHC Government 24 Chikkballapur Deccan Hospital Chintamani Private 25 Chikkballapur Devaragudipalli Government 26 Chikkballapur Dibbur PHC Government 27 Chikkballapur Dibburahalli PHC Government 28 Chikkballapur Doddaganjuru Government 29 Chikkballapur Doddatekahalli Government 30 Chikkballapur Egalitihalli Thimmasandra PHC Government 31 Chikkballapur G Maddepalli Cross PHC Government 32 Chikkballapur Gangrekaluve Government 33 Chikkballapur Ganjigunte PHC Government 34 Chikkballapur -
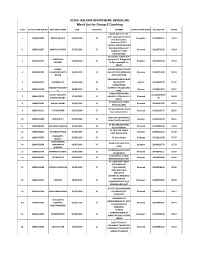
Merit List for Group-C Coaching
SOCIAL WELFARE DEPARTMENT, BENGALURU Merit List for Group-C Coaching SL NO APPLICATION REG NO APPLICANT NAME DOB CATEGORY ADDRESS EXAM CENTER NAME SSLC REG NO SCORE #6/19, 2nd cross, 4th main Jayanagar 1st block 1 1819G002896 PREETHAM M P 05/01/1994 SC Bengaluru 20100449134 92.04 east, Byrasandra, Bangalore-560011 C/O ER.S.H.RATHOD SHRI- RAM BUILDING VAJAY 2 1819G002837 AMRUTA RATHOD 27/07/1995 SC Dharwad 20110370342 90.24 NAGAR 1ST MAIN HALIYAL ROAD shreeshail y loganvi dist- SHREESHAIL vijayapur TQ- B.Bagewadi 3 1819G001395 01/06/1984 SC Belgaum 20030244284 89.33 LOGANVI At Post- manag0li Pin 586122 SURESH SURESH DASAR AT POST 4 1819G000338 YALAGURADDAPPA 25/09/1993 SC HULLUR TQ MUDDEBIHAL Dharwad 20100271498 88.29 DASAR DIST VIJAYAPUR BANASAMUDRAVILLAIGE 5 1819G003417 VINODRAJ B S 10/04/1996 SC TKHALLIPOST Mysore 20120539109 87.87 KASABAHOBLI SHARATH KUMAR H KOTHATHI VILLAGE AND 6 1819G003258 28/08/1995 SC Mysore 20110614504 87.11 P POST. RAMALINGESHWARA RAJEEV KOLLAPPA M11064375859 7 1819G001079 12/05/1995 SC NAGAR AT POST BELLATTI Dharwad 86.92 BANDIVADDARA 31 582112 AT VIRUPAPUR TANDA 8 1819G002501 ARJUN LAMANI 21/05/1993 SC Dharwad 20090387978 86.62 POST KALAKERI 5th ward behind masjid 9 1819G001631 Y TEJASHWINI 03/07/1996 SC Dharwad 20120109244 86.26 near railway station HOSA URU IBRAMPURA 10 1819G003485 NAGARAJA T 03/07/1996 SC Koppala 20120116647 86.11 VILAGE BAGEVADI POST AT DEVARAGUDIAHAL 11 1819G000032 MALLAPPA HARIJAN 21/02/1995 SC Dharwad 20110382399 85.89 POST RAYANAL AT CHATTAR TANDA 12 1819G003346 SITARAM RATHOD 07/06/1992 SC Koppala 20080161644 85.58 POST NAGALAPUR PRASHANT L 13 1819G003079 01/06/1995 SC AT post mashal Gulbarga 20120212468 85.50 JADHAV VIJAYAKUMAR dombar tot nipanal tq 14 1819G002968 YAMANAPPA 01/07/1992 SC Belgaum 20090319770 85.33 raybg DOMBAR AT NEERALAKATTI POST 15 1819G002787 SHANKAR AKKASALI 19/07/1988 SC Dharwad 20040340117 85.07 CHANDAPUR VIJAYAPURA VILLAGE 16 1819G000431 PRIYANKA K S 08/01/1991 SC Shimoga 20060610990 85.00 BENAKANAHALLI POST AT.