Differences Between Pelargonium Moniliforme (Geraniaceae) and the Closely Related P
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GTAC/CBPEP/ EU Project on Employment-Intensive Rural Land Reform in South Africa: Policies, Programmes and Capacities
GTAC/CBPEP/ EU project on employment-intensive rural land reform in South Africa: policies, programmes and capacities Municipal case study Matzikama Local Municipality, Western Cape David Mayson, Rick de Satgé and Ivor Manuel with Bruno Losch Phuhlisani NPC March 2020 Abbreviations and acronyms BEE Black Economic Empowerment CASP Comprehensive Agricultural Support Programme CAWH Community Animal Health Worker CEO Chief Executive Officer CPA Communal Property of Association CPAC Commodity Project Allocation Committee DAAC District Agri-Park Advisory Committee DAPOTT District Agri Park Operational Task Team DoA Department of Agriculture DRDLR Department of Rural Development and Land Reform DWS Department of Water and Sanitation ECPA Ebenhaeser CPA FALA Financial Assistance Land FAO Food and Agriculture Organisation FPSU Farmer Production Support Unit FTE Full-Time Equivalent GGP Gross Geographic Product GDP Gross Domestic Product GVA Gross Value Added HDI Historically Disadvantaged Individual IDP Integrated Development Plan ILO International Labour Organisation LED Local economic development LORWUA Lower Olifants Water Users Association LSU Large stock units NDP National Development Plan PDOA Provincial Department of Agriculture PGWC Provincial Government of the Western Cape PLAS Proactive Land Acquisition Strategy SDF Spatial Development Framework SLAG Settlement and Land Acquisition Grant SSU Small stock unit SPP Surplus People Project TRANCRAA Transformation of Certain Rural Areas Act WUA Water Users Association ii Table of Contents -

Munisipaliteit Bergrivier Municipality
MUNISIPALITEIT BERGRIVIER MUNICIPALITY General Valuation for 20170701 (Piketberg RD - Valuation Roll) In accordance with Section 30 of the Municipal Property Rates Act 6 of 2004 Kragtens Artikel 30 van die Munisipale Eiendomsbelastingwet 6 van 2004 Date of valuation : 20170701 © 2010 PenSoft CC (Mass Appraisal Software Solution) 2018-01-30 02:22:46 PM Valuation Roll MUNISIPALITEIT BERGRIVIER MUNICIPALITY General Valuation for 20170701 Page 2 of 63 Categories Reference Category Description AGRI Agricultural COMM Commercial INDUS Industrial INSTIT Institute MUN Municipal RES Residential PSi Public Service Infrastructure © 2010 PenSoft CC (Mass Appraisal Software Solution) 2018-01-30 02:22:46 PM Valuation Roll MUNISIPALITEIT BERGRIVIER MUNICIPALITY General Valuation for 20170701 Page 3 of 63 Geographical Area : Piketberg RD Erf No Portion Owner/s Category Address Extent Value Other Particulars 4 1 Ned Ged Kerk-Redelinghuys AGRI REDELINGHUYS 4,0000 Ha 400 000 Address :- , , , , , 4 4 Gysbert Mathys Theunis van Lill, AGRI WITTEDRIFT 1 197,1338 Ha 5 750 000 Address :- , , , , , Lill Gysbert Mathys Theunis Van 4* 5 R J F Boerdery Pty Ltd AGRI WITTEDRIFT 1 112,6351 Ha 8 345 000 Including :- Piketberg RD 4/5, Piketberg RD 4/11. Address :- , , , , , 4 5 R J F Boerdery Pty Ltd AGRI WITTEDRIFT 602,1420 Ha 0 See :- Piketberg RD 4*/5. Address :- , , , , , 4 6 Catwalk Inv 46 Pty Ltd AGRI WITTEDRIFT 1 225,6973 Ha 2 535 000 Address :- , , , , , 4 7 Villiers Philip George De AGRI WITTEDRIFT 37,1764 Ha 770 000 Address :- , , , , , 4 8 Zyl Martha Jacomina Elizabeth AGRI WITTEDRIFT 1 157,8471 Ha 4 425 000 Address :- , , , , , Van, Martha Jacomina Elizabeth van Zyl 4 9 R J F Boerdery Pty Ltd, Horsthuis AGRI WITTEDRIFT 860,5692 Ha 3 415 000 Address :- , , , , , Prop C C and other 4 10 Quick Co 14 Pty Ltd AGRI WITTEDRIFT 13,7302 Ha 840 000 Address :- , , , , , 4 11 R J F Boerdery Pty Ltd AGRI WITTEDRIFT 0 510,4931 Ha 0 See :- Piketberg RD 4*/5. -

6 the Environments Associated with the Proposed Alternative Sites
6 THE ENVIRONMENTS ASSOCIATED WITH THE PROPOSED ALTERNATIVE SITES The purpose of this section is to describe the environments associated with the proposed alternative sites. The information contained herein was extracted from the relevant specialist studies. Please refer to Section 3.5 for a list of all the relevant specialists and their fields of expertise and to Appendix E for the original specialist reports. 6.1 Brazil Site 6.1.1 Physical (a) Location The Brazil site is situated in the Kleinzee / Nolloth region of the Northern Cape, within the jurisdiction of the Nama-Khoi Municipality ( Figure 16). The site has the following co-ordinates: 29°48’51.40’’S and 17°4’42.21’’E. The Brazil site is situated approximately 500 km north of Cape Town and 100 km west-southwest of Springbok. Kleinzee is located 15 km north, Koiingnaas is 90 km south and Kamieskroon is located 90 km southeast of the Brazil site. Figure 16: Location of the proposed Brazil site in relation to the surrounding areas (Bulman, 2007) Nuclear 1 EIA: Final Scoping Report Eskom Holdings Limited 6-1 Issue 1.0 / July 2008 (b) Topography The topography in the Brazil region is largely flat, with only a gentle slope down to the coast. The coast is composed of both sandy and rocky shores. The topography is characterised by a small fore-dune complex immediately adjacent to the coast with the highest elevation of approximately nine mamsl. Further inland the general elevation depresses to about five mamsl in the middle of the study area and then gradually rises towards the east. -

A Case Study: Building Resilience in Rangelands Through a Natural Resource Management Model
A CASE STUDY: BUILDING RESILIENCE IN RANGELANDS THROUGH A NATURAL RESOURCE MANAGEMENT MODEL Ecosystem-based approaches to adaptation: strengthening the evidence and informing policy Halcyone Muller Heidi-Jayne Hawkins Sarshen Scorgie November 2019 Contents Introduction ......................................................................................................................... 4 Materials and methods .................................................................................................... 6 Climate and biophysical characteristics of the study area ............................. 6 Socio-economic characteristics of the study area ............................................. 7 Socio-economic survey .............................................................................................. 7 Biophysical study design ........................................................................................... 7 Statistics .......................................................................................................................... 8 Results .................................................................................................................................. 10 Socio-economic survey ............................................................................................. 10 Biophysical study ......................................................................................................... 11 Discussion ......................................................................................................................... -

Submission on the Issue of Relative Resourcing of Police Areas
The Commission of Inquiry into Allegations of Police Inefficient in Khayelitsha and a Breakdown of Relations between the Community and the Police in Khayelitsha Submission on the Issue of Relative Resourcing of Police Areas Jean Redpath, Community Law Centre, University of the Western Cape Summary If policing burden were distributed equally, then police human resources should be distributed through a per capita method (i.e. population size determines relative resourcing). However policing burden is not distributed equally. Any method of determining resources which deviates from the per capita method, must be checked against per capita figures for obvious anomalies. The SAPS method fails this test. A method is suggested which sees a minimum number allocated to each station, and then the remaining resources distributed according to a method which uses per capita figures to distribute most resources, but takes some account of reported crime as well as the true serious violent crime rate, as indicated by the murder rate. Introduction The issue of allocation by the state of human resources to policing is one which impinges on various constitutional rights, such as the right to safety and security of the person, dignity, life, and equality before the law, inter-related with the right not to be unfairly discriminated against. At issue is whether the distribution of state resources on policing, which impinge on the protection or realisation of these rights, is unequal to the extent that it amounts to unfair discrimination. Variations in allocations per capita are prima facie an indication of unequal distribution of resources. Where the distribution of human resources in policing is not only unequal from area to area, but areas comprising predominantly poor and black people are particularly under-resourced, indirect discrimination on protected constitutional grounds exists. -

Explore the Northern Cape Province
Cultural Guiding - Explore The Northern Cape Province When Schalk van Niekerk traded all his possessions for an 83.5 carat stone owned by the Griqua Shepard, Zwartboy, Sir Richard Southey, Colonial Secretary of the Cape, declared with some justification: “This is the rock on which the future of South Africa will be built.” For us, The Star of South Africa, as the gem became known, shines not in the East, but in the Northern Cape. (Tourism Blueprint, 2006) 2 – WildlifeCampus Cultural Guiding Course – Northern Cape Module # 1 - Province Overview Component # 1 - Northern Cape Province Overview Module # 2 - Cultural Overview Component # 1 - Northern Cape Cultural Overview Module # 3 - Historical Overview Component # 1 - Northern Cape Historical Overview Module # 4 - Wildlife and Nature Conservation Overview Component # 1 - Northern Cape Wildlife and Nature Conservation Overview Module # 5 - Namaqualand Component # 1 - Namaqualand Component # 2 - The Hantam Karoo Component # 3 - Towns along the N14 Component # 4 - Richtersveld Component # 5 - The West Coast Module # 5 - Karoo Region Component # 1 - Introduction to the Karoo and N12 towns Component # 2 - Towns along the N1, N9 and N10 Component # 3 - Other Karoo towns Module # 6 - Diamond Region Component # 1 - Kimberley Component # 2 - Battlefields and towns along the N12 Module # 7 - The Green Kalahari Component # 1 – The Green Kalahari Module # 8 - The Kalahari Component # 1 - Kuruman and towns along the N14 South and R31 Northern Cape Province Overview This course material is the copyrighted intellectual property of WildlifeCampus. It may not be copied, distributed or reproduced in any format whatsoever without the express written permission of WildlifeCampus. 3 – WildlifeCampus Cultural Guiding Course – Northern Cape Module 1 - Component 1 Northern Cape Province Overview Introduction Diamonds certainly put the Northern Cape on the map, but it has far more to offer than these shiny stones. -

Namaqualand and Challenges to the Law Community Resource
•' **• • v ^ WiKSHOr'IMPOLITICIALT ... , , AWD POLICY ANALYSi • ; ' st9K«onTHp^n»< '" •wJ^B^W-'EP.SrTY NAMAQUALAND AND CHALLENGES TO THE LAW: COMMUNITY RESOURCE MANAGEMENT AND LEGAL FRAMEWORKS Henk Smith Land reform in the arid Namaqualand region of South Africa offers unique challenges. Most of the land is owned by large mining companies and white commercial farmers. The government's restitution programme which addresses dispossession under post 1913 Apartheid land laws, will not be the major instrument for land reform in Namaqualand. Most dispossession of indigenous Nama people occurred during the previous century or the State was not directly involved. Redistribution and land acquisition for those in need of land based income opportunities and qualifying for State assistance will to some extent deal with unequal land distribution pattern. Surface use of mining land, and small mining compatible with large-scale mining may provide new opportunities for redistribution purposes. The most dramatic land reform measures in Namaqualand will be in the field of tenure reform, and specifically of communal tenure systems. Namaqualand features eight large reserves (1 200 OOOha covering 25% of the area) set aside for the local communities. These reserves have a history which is unique in South Africa. During the 1800's as the interior of South Africa was being colonised, the rights of Nama descendant communities were recognised through State issued "tickets of occupation". Subsequent legislation designed to administer these exclusively Coloured areas, confirmed that the communities' interests in land predating the legislation. A statutory trust of this sort creates obligations for the State in public law. Furthermore, the new constitution insists on appropriate respect for the fundamental principles of non-discrimination and freedom of movement. -

Integrated Development Plan (IDP) 2017-2022
HANTAM MUNICIPALITY Integrated Development Plan (IDP) 2017-2022 IDP 2021/2022 (DRAFT) March 2021- for public participation 0 “Hantam, a place of service excellence and equal opportunities creating a better life for all” 4th and Final Review of the 4th Generation Integrated Development Plan 2017-2022 Council approval: …………………….. 1 TABLE OF CONTENTS 3.6 INTERGOVERNMENTAL FORUMS ............................................ 47 3.7 MUNICIPAL DEPARTMENTS ................................................. 47 List of Tables ..................................................... 1 CHAPTER 4: PUBLIC PARTICIPATION ................. 60 List of figures ..................................................... 1 4.1 INTRODUCTION ................................................................ 60 List of Graphs/Maps .......................................... 2 4.2 SUMMARY OF WARD PRIORITIES .......................................... 60 EXECUTIVE SUMMARY ....................................... 5 CHAPTER 5: STRATEGIC AGENDA ...................... 69 CHAPTER 1: INTRODUCTION AND OVERVIEW ..... 8 5.1 INTRODUCTION ................................................................ 69 1.1 NATIONAL LEGISLATIVE FRAMEWORK ....................................... 8 5.2 SWOT ANALYSIS .............................................................. 70 1.2 PURPOSE OF THE IDP DOCUMENT ........................................... 8 5.3 VISION ........................................................................... 70 1.3 POLICY CONTEXT (HIGHER-ORDER POLICY DIRECTIVES) .................. -

Kamieskroon Bulk Water Supply, Portion 4 of Farm 445, Kamiesberg Municipality, Northern Cape
1 PALAEONTOLOGICAL HERITAGE COMMENT: KAMIESKROON BULK WATER SUPPLY, PORTION 4 OF FARM 445, KAMIESBERG MUNICIPALITY, NORTHERN CAPE John E. Almond PhD (Cantab.) Natura Viva cc, PO Box 12410 Mill Street, Cape Town 8010, RSA [email protected] January 2018 EXECUTIVE SUMMARY The overall palaeontological impact significance of the proposed Bulk Water Supply System development on Portion 4 of Farm 445 near Kamieskroon, Namaqualand, Northern Cape, is considered to be VERY LOW because the study area is underlain by unfossiliferous metamorphic basement rocks (granite-gneisses, migmatites etc) and / or mantled by superficial sediments of low palaeontological sensitivity while the development footprint is very small and in part already disturbed. It is therefore recommended that, pending the exposure of significant new fossils during development, exemption from further specialist palaeontological studies and mitigation be granted for this development. 1. PROJECT OUTLINE The proposed Bulk Water Supply System development on Portion 4 of Farm 445 near Kamieskroon, Kamiesberg Municipality, Northern Cape involves the following infrastructural components (CTS Heritage 2017; Fig. 1): • equipment for existing boreholes; • equipment for additional boreholes; • construction of a 600kl clean water storage reservoir; • installation of pipelines; • construction of a Water Treatment Works (desalination plant) and associated evaporation ponds (waste brine). 2. GEOLOGICAL CONTEXT The footprint of the proposed Bulk Water Supply System development is situated at c. 770 m asl in fairly flat, disturbed, semi-arid, rocky terrain on the outskirts of the town of Kamieskroon, some 600 m southeast of the N7 trunk road (Fig. 1). The geology of the study area near Kamieskroon is shown on the 1: 250 000 geology map 3017 Garies (Council for Geoscience, Pretoria; Fig. -
Daily Bulletin Preliminary Data for 2021/09/02 GAUTENG BRONKHORSTSPRUIT AWS 25.8 6.7 IRENE WO 25.9 10.0 JHB BOT TUINE 24.6 7
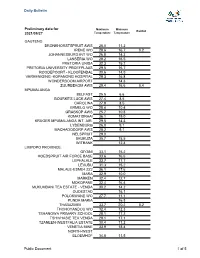
Daily Bulletin Preliminary data for Maximum Minimum Rainfall 2021/09/27 Temperature Temperature GAUTENG BRONKHORSTSPRUIT AWS 28.0 11.2 IRENE WO 28.6 16.7 0.2 JOHANNESBURG INT WO 26.8 14.3 LANSERIA WO 29.2 16.5 PRETORIA UNISA 32.3 15.2 PRETORIA UNIVERSITY PROEFPLAAS 29.5 16.7 ROODEPOORT - KLOOFENDAL 30.6 14.5 VEREENIGING -KOPANONG HOSPITAL 29.3 16.8 WONDERBOOM AIRPORT 14.3 ZUURBEKOM AWS 29.4 16.6 0.4 MPUMALANGA BELFAST 25.5 6.6 BOURKE'S LUCK AWS 27.4 8.5 CAROLINA 27.9 8.5 ERMELO WO 28.4 10.4 GRASKOP AWS 25.7 10.8 KOMATIDRAAI 36.1 19.0 KRUGER MPUMALANGA INT. AIR. 29.5 14.3 LYDENBURG 26.0 9.1 MACHADODORP AWS 28.2 9.1 NELSPRUIT 29.0 SKUKUZA 35.7 15.5 WITBANK 12.4 LIMPOPO PROVINCE GIYANI 33.1 15.2 HOEDSPRUIT AIR FORCE BASE 33.6 16.6 LEPHALALE 33.7 17.7 LEVUBU 31.3 15.2 MALALE-ESME4 ZZ2 36.1 17.6 MARA 32.9 10.0 MARKEN 32.4 12.7 MOKOPANE 32.4 16.4 MUKUMBANI TEA ESTATE - VENDA 30.2 14.2 OUDESTAD 16.1 POLOKWANE WO 27.7 11.1 PUNDA MARIA 16.8 THABAZIMBI 33.7 20.2 0.2 THOHOYANDOU WO 32.4 12.3 TSHANOWA PRIMARY SCHOOL 28.1 17.2 TSHIVHASIE TEA VENDA 29.1 17.1 TZANEEN-WESTFALIA ESTATE 30.4 11.2 VENETIA MINE 33.9 18.4 NORTH-WEST BLOEMHOF 34.8 11.5 Public Document 1 of 5 Daily Bulletin HARTEBEESPOORT DAM 32.2 14.7 KLERKSDORP 32.6 15.7 LICHTENBURG 30.7 14.4 LINDLEYSPOORT 31.5 13.2 MADIKWE GAME RESERVE 33.8 18.3 MAFIKENG WO 32.5 14.6 OTTOSDAL 32.3 13.7 PILANESBERG 32.1 15.5 RUSTENBURG 27.8 15.5 TAUNG 34.8 12.4 TOSCA 36.5 10.7 VRYBURG 33.9 10.2 FREE STATE BETHLEHEM WO 28.9 14.0 BLOEMFONTEIN - STAD 31.0 BLOEMFONTEIN WO 33.6 11.5 BOTHAVILLE - BALKFONTEIN 33.7 -

14 Northern Cape Province
Section B:Section Profile B:Northern District HealthCape Province Profiles 14 Northern Cape Province John Taolo Gaetsewe District Municipality (DC45) Overview of the district The John Taolo Gaetsewe District Municipalitya (previously Kgalagadi) is a Category C municipality located in the north of the Northern Cape Province, bordering Botswana in the west. It comprises the three local municipalities of Gamagara, Ga- Segonyana and Joe Morolong, and 186 towns and settlements, of which the majority (80%) are villages. The boundaries of this district were demarcated in 2006 to include the once north-western part of Joe Morolong and Olifantshoek, along with its surrounds, into the Gamagara Local Municipality. It has an established rail network from Sishen South and between Black Rock and Dibeng. It is characterised by a mixture of land uses, of which agriculture and mining are dominant. The district holds potential as a viable tourist destination and has numerous growth opportunities in the industrial sector. Area: 27 322km² Population (2016)b: 238 306 Population density (2016): 8.7 persons per km2 Estimated medical scheme coverage: 14.5% Cities/Towns: Bankhara-Bodulong, Deben, Hotazel, Kathu, Kuruman, Mothibistad, Olifantshoek, Santoy, Van Zylsrus. Main Economic Sectors: Agriculture, mining, retail. Population distribution, local municipality boundaries and health facility locations Source: Mid-Year Population Estimates 2016, Stats SA. a The Local Government Handbook South Africa 2017. A complete guide to municipalities in South Africa. Seventh -

Revealing the Beattie Magnetic Anomaly and the Anatomy Of
11th SAGA Biennial Technical Meeting and Exhibition Swaziland, 16 - 18 September 2009, pages 490 - 499 Revealing the Beattie Magnetic Anomaly and the anatomy of the crust of southernmost Africa: Geophysics and deep sub- surface geology where the Cape Fold Belt and Karroo Basin meet A. S. Lindeque1,2,3, M.J. de Wit4 1. Now at Alfred Wegener Institute for Polar and Marine Research, Geophysics, Building D3280, Am Alten Hafen 26, 27568 Bremerhaven, Germany, [email protected] 2. Council for Geoscience, Western Cape, P.O. Box 572, Bellville 7535, Cape Town, South Africa 3. GeoForschungsZentrum Potsdam, Section 2.2, Telegrafenberg, 14473 Potsdam, Germany 4. AEON - Africa Earth Observatory Network and Department of Geological Sciences, University of Cape Town, Rondebosch 7701, South Africa, [email protected] ABSTRACT The deep crust of the southernmost margin of Africa contains unresolved tectonic features such as the Paleozoic Cape Fold Belt (CFB), the Paleozoic-Mesozoic Karroo Basin and the largest terrestrial magnetic anomaly, the Beattie Magnetic Anomaly (BMA). Without resolving these structures, our understanding of the evolution of the southern margin will be incomplete and limited. Under the auspices of the Inkaba yeAfrica framework, several geophysical datasets were acquired from 2004 to 2007, along two transects across the margin and its unique tectonic features. This research presents a tectonic model and crustal geometry, at the centre 100 km of the western transect. The model is derived from the joint interpretation of: surface geology, aeromagnetic data, nearby deep boreholes, teleseismic receiver functions, impedance spectroscopy measurements on borehole samples, near vertical reflection seismic data (NVR), shallow P- and S-wave velocity data, wide angle refraction data and magnetotelluric data.