Quality Assurance Project Plan (H.C.S., 2006)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

From Semiahmoo First Nation to the Review Panel Re: Oral Presentation
Date: May 16, 2019 s EMIAHMOO FIRST NATION Canadian Environmental Assessment Agency Roberts Bank Terminal2 Project Public Hearing Phase Overview of Oral Hearing Submission by Semiahmoo First Nation To: Cindy Parker Review Panel Manager Roberts Bank Termina12 Project c/o Canadian Environmental Assessment Agency 160 Elgin Street, 22nd Floor Ottawa, ON K1A OH3 16049 Beach Road, Surrey, British Columbia, Canada V3Z 9R6 Tel: 604.536.3101 Fax: 604.536.6116 E-mail: [email protected] The Semiahmoo First Nation ("Semiahmoo") holds Aboriginal rights and title and exercises our rights, practices and culture throughout our Traditional Territory. Additionally, Semiahmoo exercises our rights, practices and culture throughout the broader resources area, which includes the lower Fraser River, Roberts Bank, Semiahmoo Bay, Boundary Bay, Fraser River, Nicomekl River, Serpentine River, Little Campbell River the Gulf Islands including San Juan Island, Vancouver Island, Washington State and the Salish Sea. Semiahmoo has communicated the adverse effects of the Roberts Band Terminal2 Project ("RBT2") on our Aboriginal rights and title to the project proponent and the Crown. Semiahmoo has previously demanded studies regarding cumulative effects of marine shipping in regard to the regarding the Marine Shipping Addendum and RBT2 including: • a traditional marine use study to examine the impacts on our Aboriginal rights and title; • a study of the effects of sedimentation on the foreshore of the Semiahmoo Indian Reserve lands from the tide, current and -

Mayor Wayne Baldwin Office of the Mayor
MAYOR WAYNE BALDWIN OFFICE OF THE MAYOR , WHITE ROCK, BC CANADA May 12, 2016 File No. 0220-20 Transmitted by Email: todd.stone.M [email protected] The Honourable Todd Stone, MLA Minister of Transportation and Infrastructure Room 305, Parliament Buildings Victoria, BC V8V 1X4 Dear Minister Stone: Re: Transportation of Dangerous Goods by Rail — Rail Relocation We are writing to you and to the Honourable Marc Garneau, Federal Minister of Transportation seeking consideration and support for the relocation of the Burlington Northern Santa Fe Railroad (BNSFR) from to the its current route along the coastline of the Semiahmoo Peninsula, travelling from the United States would CN/CP trackage to the Port of Vancouver, to a more direct, inland route. A relocation of this route address an extremely and increasing dangerous situation in terms of an inevitable rail derailment and other safety, environmental community concerns while providing a superior route from a time travel and operational standpoint. for a The City of White Rock and the City of Surrey are seeking partnership and funding opportunities study on options for rail relocation. predecessor, the In the early 1900’s the government granted a Right of Way for a railroad to the BNSFR’s Great Northern Railway along the coastline of the Semiahmoo Peninsula. Three communities north of the White Rock U.S. border inhabit the area along the Peninsula, the Semiahmoo First Nation, the City of of approximately (with a population of approximately 20,000) and the City of Surrey (with a population 540,000). there were between two In recent years, the volume of train traffic has increased significantly. -
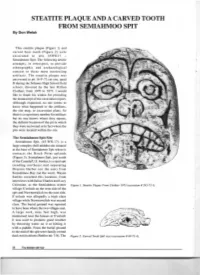
STEATITE PLAQUE and a CARVED TOOTH from SEMIAHMOO SPIT by Don Welsh
STEATITE PLAQUE AND A CARVED TOOTH FROM SEMIAHMOO SPIT By Don Welsh This steatite plaque (Figure 1) and carved bear tooth (Figure 2) were excavated in site 45WH 17 Semiahmoo Spit. The following article attempts, in retrospect, to provide ethnographic and archaeological context to these most interesting artifacts. The steatite plaque was uncovered in pit 16-F-73 cut one, quad B during the Sehome High School field school, directed by the late Milton Clothier," from 1970 to 1973. I would like to thank his widow for providing the manuscript of the excavation report. Although requested, no one seems to know what happened to the artifacts, the site map, or excavation plans. So there is a repository number for artifacts but no one knows where they repose, the definite location of the pit in which they were recovered or in fact where the pits were located within the site. The Semiahmoo Spit Site Semiahmoo Spit, ( 45-WH-17), is a large complex shell midden site situated at the base of Semiahmoo Spit where it c ontacts the Birch Point uplands (Figure 3). Semiahmoo Spit, just south of the Canada/U.S. border, is a sand spit trending northeast and separating Drayton Harbor (on the east) from Semiahmoo Bay (on the west). Wayne Suttles recorded this location, from interviews with Julius Charles and Lucy Celestine, as the Semiahmoo winter Figure 1. Steatite Plaque From Clothier 1973 (accession# 245-73-1) . village S' eel uch on the west side of the spit and Nuwnuwulich on the east side. S'eeluch was allegedly a high class village while Nuwnuwulich was second class. -

Fact Sheet Peace Arch Land Port of Entry Peace Arch Built in 1921, Renovated in 1979 and 2010 (Gross Square Footage 51,782)
GSA Northwest/Arctic Region Fact Sheet Peace Arch Land Port of Entry Peace Arch built in 1921, renovated in 1979 and 2010 (Gross square footage 51,782) 100 Peace Portal Drive Blaine, Washington 98230 Property Manager: John Shaffer Cell: (206) 369-4077 Email: [email protected] Website: http://www.gsa.gov GSA constructed the new state-of-the-art Peace Arch Land reduce water runoff. The project is also the first land port of Port of Entry (LPOE), in Blaine, Washington, in 2010. The entry to incorporate an advanced exterior lighting design to original 1920’s-era brick building underwent a major rebuild in reduce energy usage and light pollution into the atmosphere, 1979 and then again from 2007 to 2010. The new facility was and the campus incentivizes hybrid and electric vehicles by formally dedicated in March 2011. The modernization including six new charging stations, and provides them with enhanced border security, and provided a more welcoming priority parking spaces. The project has achieved a Leadership experience for visitors to both Canada and the United States. in Energy and Environmental Design (LEED) gold certification The reconstructed LPOE also features innovative technologies from the U.S. Green Building Council, the first LPOE to that will ensure taxpayers benefit from increased energy achieve this distinction in the U.S. Additionally, the new land savings. port showcases additional inspection booths to bolster security, The Peace Arch LPOE serves as the the major U.S. to Canada . The American Recovery and Reinvestment Act of 2009 border crossing where U.S. Interstate 5 ( I-5) through Seattle partially funded the recent $107 million project. -

Little Campbell River Watershed Water Quality Monitoring
MMiinniissttrryy ooff EEnnvviirroonnmmeenntt LOWER MAINLAND REGION Little Campbell River Watershed Water Quality Monitoring Y Y 2005 – 2007 T T I I L L A A U U Q Q L L A A T T N N E E M M N N O O R R I I V V N N E E Little Campbell River Watershed Water Quality Monitoring: 2005 – 2007 Prepared by: Christy Juteau Ministry of Environment Environmental Quality Section 2nd Floor, 10470 152 Street Surrey, B.C. V3R 0Y3 604-582-5200 June 2008 Preface This report is one in a series of water, groundwater, and air quality reports that are being issued by the Lower Mainland Regional Office in fiscal year 2006/07. It is the intention of the Regional Office to publish air and water quality reports on our website (http://www.env.gov.bc.ca/epd/regions/lower_mainland/index.htm) in order to provide the information to industry and local government, other stakeholders and the public at large. By providing such information in a readily understood format, and on an ongoing basis, it is hoped that local environmental quality conditions can be better understood, and better decisions regarding air and water quality management can be made. Acknowledgements This project was initiated and supported by the Shared Waters Alliance. The Ministry of Environment led and designed the project, provided funding for sampling and sample analysis, and prepared the final report. Environment Canada aided in development of the sampling plan and report review. A Rocha Canada provided support to the project through a contribution of staff time, funded through Environment Canada’s Science Horizons Youth Internship Program. -

Freshwater and Marine Fish of Boundary Bay
Freshwater and Marine Fish of Boundary Bay Compiled by Anne Murray, January 2009 The first list includes native fish that occur in freshwater streams and rivers in the Boundary Bay watershed, or in the marine waters of Boundary Bay itself, or within the Fraser River estuary (see map in A Nature Guide to Boundary Bay for area covered). The list is based on a number of sources but is almost certainly incomplete. Few studies have been done of non-salmonid fish populations in Boundary Bay waters. Some species may now be extirpated. Former or alternative names are given in brackets. A short list of introduced fish species is found on page 3. Additions, changes and suggestions for these lists are welcome. Common Name Scientific Name Western Brook Lamprey Lampetra richardsoni Pacific Lamprey Lampetra tridentata River Lamprey Lampetra ayresi White Sturgeon Acipenser transmontanus Green Sturgeon Acipenser medirostris Mountain whitefish Prosopium willamsoni Bull trout Salvelinus confluentus Dolly Varden char Salvelinus malma Coastal Cutthroat Trout Oncorhynchus clarki Steelhead Oncorhynchus mykiss Coho Salmon Oncorhynchus kisutch Chum Salmon Oncorhynchus keta Chinook Salmon Oncorhynchus tshawytscha Pink salmon Oncorhynchus gorbuscha Sockeye salmon Oncorhynchus nerka Brassy minnow Hybognathus hankinsoni Peamouth (peamouth chub) Mylocheilus caurinus Northern pikeminnow Ptycocheilus oregonensis Longnose dace Rhinichthys cataractae Nooksack dace Rhinichthys spp. Leopard dace Rhinichthys falcatus Redside shiner Richardsonius balteatus Longnose sucker -

Whatcom County Tsunami Action Plan
Whatcom County Tsunami Action Plan December 1, 2019 Whatcom County Sheriff’s Office Division of Emergency Management Whatcom County Sheriff’s Office Tsunami Action Plan 1. Contents Contents 1. CONTENTS .................................................................................................................................................... 2 2. INTRODUCTION ............................................................................................................................................ 5 3. OVERVIEW .................................................................................................................................................... 6 4. BACKGROUND .............................................................................................................................................. 7 4.1. SHORELINE AREA VULNERABILITY ........................................................................................................................ 7 4.2. ASSUMPTIONS ................................................................................................................................................ 8 5. KEY STAKEHOLDERS ...................................................................................................................................... 9 6. TSUNAMI STATEMENTS .............................................................................................................................. 12 6.1. UNITED STATES GEOLOGICAL SURVEY ............................................................................................................... -

Friends of Semiahmoo Bay Society
Friends of Semiahmoo Bay Society Marine Conservation Initiative Boundary Bay Intertidal Forage Fish Spawning Habitat Project Summary of the Project and Findings July 2006 – October 2007 Funding Provided by: Vancity EnviroFund & Vancouver Foundation Prepared for: Friends of Semiahmoo Bay Society Prepared by: Ramona C. de Graaf, BSc., MSc., Emerald Sea Research & Consulting Table of Contents Page Nos. Section One : FoSBS, Acknowledgements, Funders, Project Partners 3 Background to Project 4 Protocol Development 9 Section Two : Results 13 Field Surveys and Spawning Surveys 13 Habitat Mapping 13 Recommendations 15-22 Sampling 15 Remediation/Restoration 16 Structures 16 Seawalls and Armouring 16 Outfall pipes 17 Other structures 17 Present Sediment Conditions 18-20 Potential spawning habitat 18 Human Impact and Available Shoreline for spawning 18 Beach Sediment Enhancement 20 High value forage fish spawning habitats in Boundary Bay 21 Shade-providing vegetation 23 Section Three Beach and Station Profiles 23-51 General Overview 23 Site Description 23 Station Classification and Beach Profiles 25 Tsawwassen Beach 25 Canada/US border Station 25 Fred Gingell Park Station 28 Tsatsu Condominiums Stations 31 Delta Shores and Boundary Bay Regional Park, Delta 33 Delta Shores Station 33 Centennial Beach Station 36 Crescent Beach and Blackie Spit, Surrey, BC 39 Beecher Place Station 39 Blackie Spit Stations 42 White Rock and South Surrey, BC 43 West Beach Boat Ramp Station 44 The Rock Station 46 Little Campbell River Estuary Station 49 Peace Arch/Beach Road Stations 52 References Cited 55 Appendices Appendix A Dates and sample locations 56-75 Appendix B Field Codes 76-77 Friends of Semiahmoo Bay Society (FoSBS) FoSBS is a transboundary, project-focused stewardship group working to conserve marine, estuarine and watershed ecosystems in the lower Fraser River Delta and Boundary Bay. -

Semiahmoo Compendium
Dear Valued Guest, Welcome to Semiahmoo Resort, Golf, and Spa. We are thrilled that you have chosen to stay with us, and hope your time here will be memorable and relaxing. There are so many ways to make the most of your Semiahmoo adventure, the hard part is deciding what to do first. Our on-site amenities are sure to keep you busy, which include a full-service spa, fitness center, Packers Kitchen + Bar, indoor and outdoor tennis courts and pickle ball, hot tub, 84-degree heated pool, award-winning golf course, and an activity center providing bike, kayak, and paddleboard rentals. Should you want to seek adventure outside of the resort, the region is booming with local attractions that span from watching wildlife - onshore and offshore - to riding our iconic Plover Ferry, to taking an inspiring scenic drive and beyond. We encourage you to talk to our friendly and knowledgable Front Desk team about any type of local activity you’d like to explore. Whether you’re here for business or pleasure, you’ll find our beaches, forests, water, and mountain views remarkable. Please do take time to explore and discover the splendor of Semiahmoo. Thank you for being our guest. Nicole Newton General Manager 9565 Semiahmoo Parkway, Blaine, WA 98230 | [email protected] Health & Safety at Semiahmoo The health and safety of our guests and team members is at the heart of everything we do at Semiahmoo. In light of COVID-19, we’ve dedicated our focus on safety even further. And we’ve never been more thankful for our remote location and vast 80-acre seaside grounds with wide-open spaces, plenty of room for enjoying outdoor adventures while safely social distancing. -
Quality Assurance Project Plan
Quality Assurance Project Plan Drayton Harbor/Semiahmoo Bay Water Quality Enhancement G 1400435 November 17, 2014: Prepared by: Julie Hirsch Hirsch Consulting Services, LLC Prepared for: Nooksack Salmon Enhancement Association, Blaine Public Works Department Publication Information October, 2014 Author and Contact Information Julie Hirsch Hirsch Consulting Services, LLC (360) 510-5343 1328 Bellingham Washington 98262 Steve Winter ESA (206)789-9658 5309 Shilshole Avenue NW, Suite 200 Seattle, WA 98107 1.0 Title Page, Table of Contents, and Distribution List Quality Assurance Project Plan Drayton Harbor/Semiahmoo Bay Water Quality Enhancement Grant 1400435 November 2014 Approved by: Signature: Date: Bill Bullock, Blaine Public Works Department Assistant Director, Client Signature: Date: Ravyn Whitewolf, Blaine Public Works Department Director, Client’s Supervisor Signature: Annitra Ferderer Date: Annitra Ferderer, Nooksack Salmon Enhancement Association, Client, Field Manager Signature: Date: 11/13/2014 Julie Hirsch, Author/Project Manager Signature: Date: Steve Winter, ESA, Author/Principal Investigator Signature: Date: N/A, Author’s Supervisor Signature: Date: Stephanie Bailey, EPA Region 10 Laboratory Microbiologist Signature: Date: Frank Arnett, Blaine Public Works, Lighthouse Point Water Reclamation Facility Lab Director Signature: Date: 11/13/2014 Julie Hirsch, Organization Quality Assurance Officer/Manager Drayton Harbor/Semiahmoo Bay Water Quality Enhancement HCS & ESA 10/2014 Page i Signature: Date: Kim Levesque, Ecology Project -

Coastal Flood Adaptation Strategy Final Report November 2019
NOVEMBER 2019 ACKNOWLEDGEMENTS The City of Surrey CFAS project team would like to thank the South Port Strata, Anderson Strata, Surrey Board of Trade, 2,000+ residents, business owners, and other stakeholders Fraser Valley Real Estate Board, Insurance Bureau of and partners who participated in the CFAS project, including: Canada, Delta Farmers’ Institute, Mud Bay Dyking District, West Coast Environmental Law, Lower Fraser Fisheries · Local and Regional Governments: Semiahmoo First Alliance Nation, City of White Rock, City of Delta, Metro Vancouver · Academic: UBC School of Architecture and Landscape · Agencies, Ministries, Crown Corporations, and Architecture and School of Community and Regional Utilities: BC Hydro, Department of Fisheries and Oceans Planning, ACT SFU, Kwantlen Park University, University (DFO), Ministry of Agriculture, Ministry of Transportation of the Fraser Valley and Infrastructure, Ministry of Forests, Lands and Natural Resource Operations and Rural Development, Ministry As a community-driven, participatory project, their time, of Municipal Affairs and Housing, BC Ambulance Service, contributions and unique perspectives helped us create the Fraser Basin Council, BC Climate Action Secretariat, adaptation approaches and pathways this strategy outlines Emergency Management BC (EMBC), Provincial and supported the community conversations and learning Agricultural Land Commission (ALC), FortisBC, Telus, SRY that was a hallmark to the initiative. Thank you. Rail Link, BNSF Railway, BC Rail, Consulate General of the Netherlands in Vancouver This document features photos from a photo contest that was conducted as part of the CFAS project. The · Organizations: Ducks Unlimited Canada, Friends of #SurreyCoastal photo contest asked people to share pictures Semiahmoo Bay Society, Stewardship Centre for B.C. of their favourite places and activities along Surrey’s coastline (Green Shores), Little Campbell Watershed Society, and attracted 220+ submissions. -

Point Roberts, Boundary Bay, San Juan & Gulf Islands Archipelago
Uo So NATIONAL PARK SERVICE PARKS CANADA AN INVENTORY OF INTERNATIONAL PARK POSSIBILITIES: POINT ROBERTS, BOUNDARY BAY, SAN JUAN AND GULF ISLANDS ARCHIPEIAGO A JOINT REPORT PREPARED FOR THE INTERNATIONAL POINT ROBERTS BOARD AUGUST 1973 CONTENTS INTRODUCTION ADMINISTRATION AND MANAGEMENT Origin and Scope of the Study 1 Alternative Administrative Actions 80 The Concept of an International Park 3 Guiding Principles and Management Objectives 80 Suggested Administrative Actions 82 PARK RESOURCES OF THE REGION Management Guidelines: Point Roberts and Environs 83 Overview of the Study Region 4 Potential Park Resources: Canada 7 MAPS AND FIGURES Potential Park Resources: United States 10 The Study Region Resource Inventory 17 Potential Park Resources TRENDS AND PATTERNS Boating Patterns Introduction 38 Impending Land Use Growing Recreation Demands 38 International Park Alternatives Land-Use Trends so Park Concept A3 CONCEPTUAL PARK ALTERNATIVES Introduction 60 Park Concepts 62 ORIGIN AND SCOPE OF STUDY are no other focal points of park interest elsewhere in the Puget The International Point Roberts Board at its meeting with the Sound-Strait of Georgia region deserving separate study. The International Joint Commission on April 11, 1972, suggested terms of reference for accomplishing the study were developed and that the U. S. National Park Service and the Canadian National agreed to by the U. S .- National Park Service and Canadian National and Historic Parks Branch be asked to investigate the park and Historic Parks Branch on December 12, 1972. potentials of Point Roberts. In line with the expressed intent of the Point Roberts Board, The commission authorized the board to request the U.