Open Payments Annual Report to Congress Fiscal Year 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fully Human Domain Antibody Therapeutics: the Best of Both Worlds
Drug Discovery Fully Human Domain Antibody Therapeutics: The Best of Both Worlds By combining the therapeutic benefits of small molecule drugs with those of fully human antibodies, Domain Antibodies are expected to have strong therapeutic and commercial potential. By Robert Connelly at Domantis Robert Connelly is Chief Executive Officer of Domantis. He has over 22 years’ commercial experience of the life science sector, including that gained in the fields of diagnostics, drug discovery technologies and antibody therapeutics. Prior to joining Domantis, he was CEO of Veritas Pharmaceuticals (Los Angeles, USA), an in vivo imaging start-up company. He spent over five years with IGEN International, latterly as Senior Vice President and General Manager, Life Sciences, where he took part in the company’s IPO and financing rounds, raising $130 million. The first 11 years of his career were spent at Abbott Laboratories in sales, marketing and management positions. Domain Antibodies (dAbs) are the smallest functional variable regions of either the heavy (VH) or light (VL) binding units of antibodies. At Domantis, we are chains of human antibodies. Domantis scientists applying our proprietary know-how in dAbs to deliver have used the variable domains sequences of human human therapies that address large, unmet medical antibodies to create a series of large and highly needs in areas such as inflammation, cancer and functional libraries of fully human dAbs, with each autoimmune diseases. Three and a half years after library comprising at least 1010 different dAbs. The opening our laboratories, we have a dozen proprietary dAbs selected from these libraries are both specific therapeutic programmes underway, and an additional for their biological target and are well folded and eight therapeutic programmes with partners. -

Chaotic Descriptor Table
Castle Oldskull Supplement CDT1: Chaotic Descriptor Table These ideas would require a few hours’ the players back to the temple of the more development to become truly useful, serpent people, I decide that she has some but I like the direction that things are going backstory. She’s an old jester-bard so I’d probably run with it. Maybe I’d even treasure hunter who got to the island by redesign dungeon level 4 to feature some magical means. This is simply because old gnome vaults and some deep gnome she’s so far from land and trade routes that lore too. I might even tie the whole it’s hard to justify any other reason for her situation to the gnome caves of C. S. Lewis, to be marooned here. She was captured by or the Nome King from L. Frank Baum’s the serpent people, who treated her as Ozma of Oz. Who knows? chattel, but she barely escaped. She’s delirious, trying to keep herself fed while she struggles to remember the command Example #13: word for her magical carpet. Malamhin of the Smooth Brow has some NPC in the Wilderness magical treasures, including a carpet of flying, a sword, some protection from serpents thingies (scrolls, amulets?) and a The PCs land on a deadly magical island of few other cool things. Talking to the PCs the serpent people, which they were meant and seeing their map will slowly bring her to explore years ago and the GM promptly back to her senses … and she wants forgot about it. -

UNIVERSIDADE ESTADUAL DE CAMPINAS Faculdade De Educação Física
UNIVERSIDADE ESTADUAL DE CAMPINAS Faculdade de Educação Física HÉLIO JOSÉ COELHO JUNIOR FRAILTY: PREVALENCE, ASSOCIATED FACTORS AND TREATMENT THROUGH RESISTANCE TRAINING FRAGILIDADE: PREVALÊNCIA, FATORES ASSOCIADOS E TRATAMENTO ATRAVÉS DO TREINAMENTO DE FORÇA CAMPINAS 2019 HÉLIO JOSÉ COELHO JUNIOR FRAILTY: PREVALENCE, ASSOCIATED FACTORS AND TREATMENT THROUGH RESISTANCE TRAINING FRAGILIDADE: PREVALÊNCIA, FATORES ASSOCIADOS E TRATAMENTO ATRAVÉS DO TREINAMENTO DE FORÇA Thesis presented to the Faculty of Physical Education of the University of Campinas in partial fulfillment of the requirements for the degree of Doctor, in the area of adapted physical activity. Supervisor: MARCO CARLOS UCHIDA Co-supervisor: BRUNO RODRIGUES ESTE TRABALHO CORRESPONDE À VERSÃO FINAL DA TESE DEFENDIDA PELO ALUNO HÉLIO JOSÉ COELHO JUNIOR, E ORIENTADA PELO PROF. DR. MARCO CARLOS UCHIDA CAMPINAS 2019 Comissão Examinadora Marco Carlos Uchida (Presidente) Emanuele Marzetti Reury Frank Pereira Bacurau Eduardo Lusa Cadore Lígia de Moraes Antunes Correa A Ata da defesa com as respectivas assinaturas dos membros encontra-se no SIGA/Sistema de Fluxo de Dissertação/Tese e na Secretaria do Programa da Unidade. AGRADECIMENTOS Prof. Dr. Marco Carlos Uchida. Sensei, há quase 10 anos o senhor me acolheu como um filho e me ajudou na escolha dos caminhos ao longo dessa jornada. Que orgulho em dizer que o senhor é, não só o meu orientador, mas o meu mentor: um pai científico, seja lá o que isso queira dizer. Obrigado por todo o companheirismo e carinho, por ter aceitado dividir comigo momentos em que o senhor podia apenas se abster. Obrigado pelas conversas incansáveis e por me presentear com uma amizade muita sincera, aceitando as minhas limitações. -

The Year of Investment, Innovation and Implementation
2017 the year of investment, innovation and implementation 2017 annual report healthcare businesswomen’s association table of contents LETTER FROM CHAIR 1 INVESTMENT 2017 BY THE NUMBERS 2 MEMBERSHIP 2 CHAPTER BREAKDOWN 2 40 YEARS OF MILESTONES 2 MEMBER/VOLUNTEER SURVEY RESULTS 3 FINANCIALS 4 INNOVATION AND IMPLEMENTATION 2017 FLAGSHIP EVENTS 7 WOMAN OF THE YEAR 8 LUMINARIES 10 RISING STARS 11 ANNUAL CONFERENCE 13 ACE AWARDS 14 THE GENDER PARITY COLLABORATIVE CLICK HERE HBA “NOW” THE NEW OPERATING MODEL CLICK HERE IN APPRECIATION—WE COULDN’T DO IT WITHOUT YOU 2017 CORPORATE PARTNERS 15 2017 SPONSORS 16 2017 MEDIA PARTNERS 17 HBA ADVISORY BOARD 18 HBA BOARD OF DIRECTORS 19 LETTER FROM CEO LAURIE COOKE 20 HBA 2017 II annual report letter from the chair In my long career as a healthcare executive, I have seen firsthand the benefits of diversity in leadership time and again. From gender to ethnicity to age and other factors, the best engagement and the most powerful results always arise when there is a diversity of perspectives in the room. This is why I joined the HBA board. This year marks the 40th anniversary of the organization’s founding. And though I am a relatively new member, I quickly became a believer in the HBA’s rich history and deep commitment to both the advancement of women individually, and to the achievement of gender parity in healthcare overall. This is also why I am proud of the HBA’s aggressive—but achievable—strategic plan to move the needle on gender parity through partnership by 2020. -

ANNUAL and SUSTAINABILITY REPORT 2017 Forward-Looking Statements This Report Includes Forward-Looking State- Ments
Building on our strengths Building ANNUAL AND SUSTAINABILITY REPORT 2017 Forward-looking statements This report includes forward-looking state- ments. Actual results may differ from those stated. Internal factors such as the successful management of research programmes and intellectual property rights may affect future results. There are also external conditions such as the economic climate, political changes and competing research programmes that may affect Sobi’s results. Sobi™ is an international biotechnology company dedicated to rare diseases. Our mission is to transform the lives of people with rare diseases by providing inno vative therapies in our focus areas.” Guido Oelkers, CEO CONTENTS Contents This is Sobi .......................................................................4 The year in brief ..........................................................6 INTERVIEW Interview with the CEO and Chairman ......................8 CEO and Chairman Strategy .....................................................................10 8 Sobi operates throughout the entire value chain, from early research, over to clinical development and into the commercial market. Business areas ...............................................................16 “We can add significant value to our partnerships by leveraging all Haemophilia ..............................................................16 our com petencies, making us the partner of choice for a range of Specialty Care ...........................................................22 companies -

Guidelines with Regard to the Composition, Calculation and Management of the Index
INDEX METHODOLOGY Solactive Pharma Breakthrough Value Index Version 2.1 dated September 03, 2020 Contents Important Information 1. Index specifications 1.1 Short Name and ISIN 1.2 Initial Value 1.3 Distribution 1.4 Prices and Calculation Frequency 1.5 Weighting 1.6 Index Committee 1.7 Publication 1.8 Historical Data 1.9 Licensing 2. Composition of the Index 2.1 Selection of the Index Components 2.2 Ordinary Adjustment 2.3 Extraordinary Adjustment 3. Calculation of the Index 3.1 Index Formula 3.2 Accuracy 3.3 Adjustments 3.4 Dividends and other Distributions 3.5 Corporate Actions 3.6 Correction Policy 3.7 Market Disruption 3.8 Consequences of an Extraordinary Event 4. Definitions 5. Appendix 5.1 Contact Details 5.2 Calculation of the Index – Change in Calculation Method 2 Important Information This document (“Index Methodology Document”) contains the underlying principles and regulations regarding the structure and the operating of the Solactive Pharma Breakthrough Value Index. Solactive AG shall make every effort to implement regulations. Solactive AG does not offer any explicit or tacit guarantee or assurance, neither pertaining to the results from the use of the Index nor the Index value at any certain point in time nor in any other respect. The Index is merely calculated and published by Solactive AG and it strives to the best of its ability to ensure the correctness of the calculation. There is no obligation for Solactive AG – irrespective of possible obligations to issuers – to advise third parties, including investors and/or financial intermediaries, of any errors in the Index. -

Investor Presentation March 19, 2019
Transaction Update INVESTOR PRESENTATION MARCH 19, 2019 1 Important Information For Investors And Stockholders This communication does not constitute an offer to sell or the solicitation of an offer to buy any securities or a solicitation of any vote or approval. It does not constitute a prospectus or prospectus equivalent document. No offering of securities shall be made except by means of a prospectus meeting the requirements of Section 10 of the U.S. Securities Act of 1933, as amended. In connection with the proposed transaction between Bristol-Myers Squibb Company (“Bristol-Myers Squibb”) and Celgene Corporation (“Celgene”), on February 1, 2019, Bristol-Myers Squibb filed with the Securities and Exchange Commission (the “SEC”) a registration statement on Form S-4, as amended on February 1, 2019 and February 20, 2019, containing a joint proxy statement of Bristol-Myers Squibb and Celgene that also constitutes a prospectus of Bristol- Myers Squibb. The registration statement was declared effective by the SEC on February 22, 2019, and Bristol-Myers Squibb and Celgene commenced mailing the definitive joint proxy statement/prospectus to stockholders of Bristol-Myers Squibb and Celgene on or about February 22, 2019. INVESTORS AND SECURITY HOLDERS OF BRISTOL-MYERS SQUIBB AND CELGENE ARE URGED TO READ THE DEFINITIVE JOINT PROXY STATEMENT/PROSPECTUS AND OTHER DOCUMENTS FILED OR THAT WILL BE FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY BECAUSE THEY CONTAIN OR WILL CONTAIN IMPORTANT INFORMATION. Investors and security holders will be able to obtain free copies of the registration statement and the definitive joint proxy statement/prospectus and other documents filed with the SEC by Bristol-Myers Squibb or Celgene through the website maintained by the SEC at http://www.sec.gov. -

Lilly to Acquire Imclone Systems in $6.5 Billion Transaction
Lilly to Acquire ImClone Systems in $6.5 Billion Transaction Creates a Global Leader in Oncology Biopharmaceuticals Boosts Oncology Pipeline With Up to Three Promising Targeted Therapies in Phase III in 2009 INDIANAPOLIS and NEW YORK, Oct 06, 2008 /PRNewswire-FirstCall via COMTEX News Network/ -- Eli Lilly and Company (NYSE: LLY) and ImClone Systems Inc. (Nasdaq: IMCL) today announced that the boards of directors of both companies have approved a definitive merger agreement under which Lilly will acquire ImClone through an all cash tender offer of $70.00 per share, or approximately $6.5 billion. The offer represents a premium of 51 percent to ImClone's closing stock price on July 30, 2008, the day before an acquisition offer for ImClone was made public. ImClone's board recommends that ImClone's shareholders tender their shares in the tender offer. Additionally, certain entities associated with ImClone's chairman, Carl C. Icahn, holding approximately 14 percent of ImClone's outstanding common stock, have agreed to tender their shares in the tender offer. This strategic combination will create one of the leading oncology franchises in the biopharmaceutical industry, offering both targeted therapies and oncolytic agents along with a pipeline spanning all phases of clinical development. The combined oncology portfolio will target a broader array of solid tumor types including lung, breast, ovarian, colorectal, head and neck, and pancreatic, positioning Lilly to pursue treatments of multiple cancers. Combining with ImClone will further strengthen Lilly's growing portfolio of first-in-class and best-in-class pharmaceutical products, enabling Lilly to better support oncologists, with the ultimate goal of delivering better outcomes for cancer patients. -

11/09/2016 Provider Subsystem Healthcare and Family Services Run Time: 20:25:21 Report Id 2794D051 Page: 01
MEDICAID SYSTEM (MMIS) ILLINOIS DEPARTMENT OF RUN DATE: 11/09/2016 PROVIDER SUBSYSTEM HEALTHCARE AND FAMILY SERVICES RUN TIME: 20:25:21 REPORT ID 2794D051 PAGE: 01 NUMERIC COMPLETE LIST OF PHARMACEUTICAL LABELERS WITH SIGNED REBATE AGREEMENTS IN EFFECT AS OF 01/01/2017 NDC NDC PREFIX LABELER NAME PREFIX LABELER NAME 00002 ELI LILLY AND COMPANY 00145 STIEFEL LABORATORIES, INC, 00003 E.R. SQUIBB & SONS, LLC. 00149 WARNER CHILCOTT PHARMACEUTICALS INC. 00004 HOFFMANN-LA ROCHE 00168 E FOUGERA AND CO. 00006 MERCK & CO., INC. 00169 NOVO NORDISK, INC. 00007 GLAXOSMITHKLINE 00172 IVAX PHARMACEUTICALS, INC. 00008 WYETH LABORATORIES 00173 GLAXOSMITHKLINE 00009 PFIZER, INC 00178 MISSION PHARMACAL COMPANY 00013 PFIZER, INC. 00182 GOLDLINE LABORATORIES, INC. 00015 MEAD JOHNSON AND COMPANY 00185 EON LABS, INC. 00023 ALLERGAN INC 00186 ASTRAZENECA LP 00024 SANOFI-AVENTIS, US LLC 00187 VALEANT PHARMACEUTICALS NORTH AMERICA 00025 PFIZER, INC. 00206 LEDERLE PIPERACILLIN 00026 BAYER HEALTHCARE LLC 00224 KONSYL PHARMACEUTICALS, INC. 00029 GLAXOSMITHKLINE 00225 B. F. ASCHER AND COMPANY, INC. 00032 SOLVAY PHARMACEUTICALS, INC. 00228 ACTAVIS ELIZABETH LLC 00037 MEDA PHARMACEUTICALS, INC. 00245 UPSHER-SMITH LABORATORIES, INC. 00039 SANOFI-AVENTIS, US LLC 00258 FOREST LABORATORIES INC 00046 AYERST LABORATORIES 00259 MERZ PHARMACEUTICALS 00049 PFIZER, INC 00264 B. BRAUN MEDICAL INC. 00051 UNIMED PHARMACEUTICALS, INC 00281 SAVAGE LABORATORIES 00052 ORGANON USA INC. 00299 GALDERMA LABORATORIES, L.P. 00053 CSL BEHRING 00300 TAP PHARMACEUTICALS INC 00054 ROXANE LABORATORIES, INC. 00310 ASTRAZENECA LP 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 00327 GUARDIAN LABS DIV UNITED-GUARDIAN INC 00062 ORTHO MCNEIL PHARMACEUTICALS 00338 BAXTER HEALTHCARE CORPORATION 00064 HEALTHPOINT, LTD. 00378 MYLAN PHARMACEUTICALS, INC. -
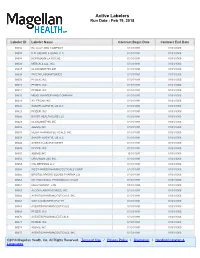
Active Labelers Run Date : Feb 19, 2018
Active Labelers Run Date : Feb 19, 2018 Labeler ID Labeler Name Contract Begin Date Contract End Date 00002 ELI LILLY AND COMPANY 01/01/1991 01/01/3000 00003 E.R. SQUIBB & SONS, LLC. 01/01/1991 01/01/3000 00004 HOFFMANN-LA ROCHE 01/01/1991 01/01/3000 00006 MERCK & CO., INC. 01/01/1991 01/01/3000 00007 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00008 WYETH LABORATORIES 01/01/1991 01/01/3000 00009 PFIZER, INC 01/01/1991 01/01/3000 00013 PFIZER, INC. 01/01/1991 01/01/3000 00014 PFIZER, INC 01/01/1991 01/01/3000 00015 MEAD JOHNSON AND COMPANY 01/01/1991 01/01/3000 00023 ALLERGAN INC 01/01/1991 01/01/3000 00024 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00025 PFIZER, INC. 01/01/1991 01/01/3000 00026 BAYER HEALTHCARE LLC 01/01/1991 01/01/3000 00029 GLAXOSMITHKLINE 01/01/1991 01/01/3000 00032 ABBVIE INC. 01/01/1991 01/01/3000 00037 MEDA PHARMACEUTICALS, INC. 01/01/1991 01/01/3000 00039 SANOFI-AVENTIS, US LLC 01/01/1991 01/01/3000 00046 AYERST LABORATORIES 01/01/1991 01/01/3000 00049 PFIZER, INC 01/01/1991 01/01/3000 00051 ABBVIE INC 10/01/1997 01/01/3000 00052 ORGANON USA INC. 01/01/1991 01/01/3000 00053 CSL BEHRING LLC 01/01/1991 01/01/3000 00054 WEST-WARD PHARMACEUTICALS CORP. 01/01/1991 01/01/3000 00056 BRISTOL-MYERS SQUIBB PHARMA CO. 01/01/1991 01/01/3000 00062 ORTHO MCNEIL PHARMACEUTICALS 01/01/1991 01/01/3000 00064 HEALTHPOINT, LTD. -

Boot Camp Roster Through July 2015
Participants in our GMP Boot Camps have come from these companies AB Biotechnologies Irisys, Inc. Abbott Vascular IVX Animal Health Acacia Biomedical Pte Ltd. J & J Packaging Actavis Pharmaceuticals Johnson Matthey Adhesives Research Karl Storz Endovision AHC Products, Inc. Kenco Management Services Alcon Labs King Pharmaceuticals Alk-Abello Source Materials Kleen Test Products Allergan, Inc. Kosan Amax Nutrasource KV Pharmaceutical American Red Cross La Jolla Pharmaceuticals Aptalis Pharmaceuticals Label World Artes Medical Labortorios Sophia Astellas Pharma Technologies Leica Microsystems Astra Zeneca Mayo Clinic Atex Technologies, Inc. Medicomp Inc. Auer Precision Co. Merck Avail Medical Metor-Logics Avema Pharma Solutions NBTY B. Braun Medical Nexgen Pharma BASF Nitto Denko Technical Inc. Baxa Corp. Norwich Pharmaceuticals Baxter Healthcare Novartis Animal Health US, Inc. Bayer Nuskin Enterprises Beckman Coulter Nutri-Mack Beiersdorf Inc. NuWorld Beauty Bend Research Oakley Biovail Ohm Laboratories Block Medical Organogenesis Inc. Boston Scientific Pacira Pharmaceuticals Bradley Corp. Palm Beach Pharmaceuticals Bristol-Myers Squibb Par Pharmaceutical Inc. CAMAG Pennakem LLC Carl Zeiss Meditec, Inc. Peter Cremer North America Cepheid Pfizer Church & Dwight Co. Philip Morris USA Cornerstone Research and Development Philliips Medisize GMP Training Systems, Inc. P.O. Box 2585 Orange, CA 92859 714-289-1233 www.GMPTrainingSystems.com Participants in our GMP Boot Camps have come from these companies CR Bard PL Developments Daiichi Sankyo Pharma Development Protab Laboratories Danisco USA Purdue Pharmaceuticals Dial Eisai Qm5 DSI, Inc. Ranbaxy Labs DSM Pharmaceuticals Raptor Pharmaceuticals Earthwise Nutritionals Regeneron Pharmaceuticals Edwards Lifesciences RJ Reynolds Co. Elan Robinson Pharma Co. Endosonics Romark Labs Eurand, Inc. Rosendin Electric Flextronics, Inc. Sanofi aventis FMI Schick, division of Energizer Forest Labs Sensible Organics Foster Corporation Shire Pharmaceuticals Garmon Corp. -

Zeus in the Greek Mysteries) and Was Thought of As the Personification of Cyclic Law, the Causal Power of Expansion, and the Angel of Miracles
Ζεύς The Angel of Cycles and Solutions will help us get back on track. In the old schools this angel was known as Jupiter (Zeus in the Greek Mysteries) and was thought of as the personification of cyclic law, the Causal Power of expansion, and the angel of miracles. Price, John Randolph (2010-11-24). Angels Within Us: A Spiritual Guide to the Twenty-Two Angels That Govern Our Everyday Lives (p. 151). Random House Publishing Group. Kindle Edition. Zeus 1 Zeus For other uses, see Zeus (disambiguation). Zeus God of the sky, lightning, thunder, law, order, justice [1] The Jupiter de Smyrne, discovered in Smyrna in 1680 Abode Mount Olympus Symbol Thunderbolt, eagle, bull, and oak Consort Hera and various others Parents Cronus and Rhea Siblings Hestia, Hades, Hera, Poseidon, Demeter Children Aeacus, Ares, Athena, Apollo, Artemis, Aphrodite, Dardanus, Dionysus, Hebe, Hermes, Heracles, Helen of Troy, Hephaestus, Perseus, Minos, the Muses, the Graces [2] Roman equivalent Jupiter Zeus (Ancient Greek: Ζεύς, Zeús; Modern Greek: Δίας, Días; English pronunciation /ˈzjuːs/[3] or /ˈzuːs/) is the "Father of Gods and men" (πατὴρ ἀνδρῶν τε θεῶν τε, patḕr andrōn te theōn te)[4] who rules the Olympians of Mount Olympus as a father rules the family according to the ancient Greek religion. He is the god of sky and thunder in Greek mythology. Zeus is etymologically cognate with and, under Hellenic influence, became particularly closely identified with Roman Jupiter. Zeus is the child of Cronus and Rhea, and the youngest of his siblings. In most traditions he is married to Hera, although, at the oracle of Dodona, his consort is Dione: according to the Iliad, he is the father of Aphrodite by Dione.[5] He is known for his erotic escapades.