8 Subjective Refraction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prevention of Traumatic Corneal Ulcer in South East Asia
FROM OUR SOUTH ASIA EDITION Prevention of traumatic corneal ulcer in South East Asia S C AE Srinivasan/ (c)M Country Principal Investigator and Lead Principal Investigator with village health workers in Bhutan Dr. M. Srinivasan ciasis, and leprosy, are declining, and (VVHW) of the Government were utilized Director Emeritus, Aravind Eye Care, soon the majority of corneal blindness will to identify ocular injury and treat corneal Madurai, Tamil Nadu India. be due to microbial keratitis. Most abrasion corneal ulcers occur among agricultural Myanmar: Village Health Workers (VHW) workers in developing countries following of the health department Introduction corneal abrasion. India: paid village volunteers were utilized Corneal ulceration is a leading cause of Several non-randomized prevention visual impairment globally, with a dispro- studies conducted before 2000 Inclusion criteria 2 portionate burden in developing (Bhaktapur Eye Study) and during 2002 • Resident of study area countries. It was estimated that 6 million to 2004 in India, Myanmar, and Bhutan • Corneal abrasion after ocular injury, corneal ulcers occur annually in the ten by World Health Organization(WHO), have confirmed by clinical examination with countries of South East Asia Region suggested that antibiotic ointment fluorescein stain and a blue torch encompassing a total population of 1.6 applied promptly after a corneal abrasion • Reported within 48 hours of the injury billion.1 While antimicrobial treatment is could lower the incidence of ulcers, • Subject aged >5 years of age generally effective in treating infection, relative to neighbouring or historic “successful” treatment is often controls.3-4 Prevention of traumatic Exclusion criteria associated with a poor visual outcome. -

Physical Eye Examination
Physical Eye Examination Kaevalin Lekhanont, MD Department of Ophthalmology Ramathibodi Hospp,ital, Mahidol Universit y Outline • Visual acuity (VA) testing – Distant VA test – Pinhole test – Near VA test • Visual field testing • Record and interpretations Outline • Penlight examination •Swingggping penli ght test • Direct ophthalmoscopy – Red reflex examination • Schiotz tonometry • RdditttiRecord and interpretations Conjunctiva, Sclera Retina Cornea Iris Retinal blood vessels Fovea Pupil AtAnteri or c ham ber Vitreous Aqueous humor Lens Optic nerve Trabecular meshwork Ciliary body Choriod and RPE Function evaluation • Visual function – Visual acuity test – Visual field test – Refraction • Motility function Anatomical evaluation Visual acuity test • Distant VA test • Near VA test Distance VA test Snellen’s chart • 20 ฟุตหรือ 6 เมตร • วัดที่ละขาง ตาขวากอนตาซาย • ออานทละตาานทีละตา แถวบนลงลแถวบนลงลางาง • บันทึกแถวลางสุดที่อานได Pinhole test VA with pinhole (PH) Refractive error emmetitropia myypopia hyperopia VA record 20/200 ผูปวยสามารถอานต ัวเลขทมี่ ี ขนาดใหญขนาดใหญพอทคนปกตพอที่คนปกติ สามารถอานไดจากท ี่ระยะ 200 ฟตฟุต แตแตผผปูปวยอานไดจากวยอานไดจาก ที่ระยะ 20 ฟุต 20/20 Distance VA test • ถาอานแถวบนสุดไไไมได ใหเดินเขาใกล chthart ทีละกาวจนอานได (10/200, 5/200) • Counting finger 2ft - 1ft - 1/2ft • Hand motion • Light projection • Light perception • No light perception (NLP) ETDRS Chart Most accurate Illiterate E chart For children age ≥ 3.5 year Near VA test Near chart •14 นวิ้ หรอื 33 เซนตเมตริ • วัดที่ละขาง ตาขวากอนตาซาย • อานทีละตา แถวบนลงลาง -
Examination of The
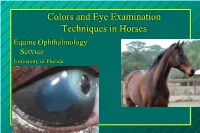
Colors and Eye Examination Techniques in Horses Equine Ophthalmology Service University of Florida There are really only 3 ophthalmic diseases!! 1. Corneal ulcers 2. Uveitis 3. Everything else!! Heine C-002-14-400 Heine C-002.14.602 Obvious Things Just stand back and look at the symmetry – Lashes – Discomfort and squinting – Tearing – Colors – Pupil – Clarity of cornea and lens – The normal eye is “shiny” – Anatomy: anterior to posterior 95677 Champagne RMH Lashes pointing down can be early Lashes sign of eye pain (ponies can look through their lashes) Nuclear Sclerosis Ocular Discomfort Level 107656 Dusty UF Res Corneal “Colors” White cornea: abscess or necrosis Blue cornea: edema Red cornea: vessels – Superficial (tree-like) and deep vessels (brush). – Note intensity of the red Dark is thin Shiny is thin Vascular Patterns Redness – Very Red – Pale Symmetry – Asymmetry 189711 154841 194013 Asymmetrical vascularization Callie Edema Corneal Haze – Endothelial – Uveitis Subepithelial scar Corneal abrasion/ulcer Subepithelial inflitrate – Immune mediated Fly – Fungal Mount Oakely IMMK Shooter Epithelial edema Sunshine Chief Barber IMMK Jupiter: DSA Chronic Recurrent Deep Immune Mediated Keratitis Green fluid filled lacunae form in the stroma “Alexiej” May SEK: fungi Candleabra SEK Pupil Size Dilated – Glaucoma – Retinal Disease – Optic Nerve Disease Miotic – Uveitis Deep corneal scrapings at the edge of the ulcer to detect bacteria and fungal hyphae Superficial swabbing cannot be expected to yield microbes in a high percentage of cases. Scrape with handle end of scalpel blade. Fluorescein: Every eye exhibiting signs of pain should be stained!! – Detects a corneal epithelial defect or “ulcer”. – Cobalt blue filter aids detection of abrasions. -

Sequence of Examination Step I Note the General Appearance As You Take the History and When Initiating the Physical Examination, Usually with the Patient Sitting
Introduction As well as history taking, Physical Examination is an important part of patient management. The goal of the P/E is to obtain valid information concerning the health of the patient. The examiner must be able to identify , analyze and synthesize the accumulated information into a comprehensive assessment. The four principles of physical examination are the following: 1. Inspection can provide an enormous amount of information. You must train themselves to look at the body using a systematic approach. 2. Palpation is the use of the tactile sense to determine the characteristics of an organ system. 3. Percussion relates to the tactile sensation and sound produced when a sharp blow is struck to an area being examined. This provides valuable information about the structure of the underlying organ or tissue. 4. Auscultation involves listening to sounds produced by internal organs. This technique furnishes information about an organ’s pathophysiology. To achieve competence in these procedures, the student must, "teach the eye to see, the finger to feel and the ear to hear" . Note: These four principles have different value in different systems. For example, Inspection is more useful in general appearance, Palpation in abdominal examination, Percussion in organ size evaluation, and Auscultation in heart examination. In abdominal examination, Auscultation is first, since performing percussion or palpation, may alter the frequency of bowel sounds. Preparing for the physical examination - Reflect on your approach to the patient - Decide on the scope of the examination - Adjust the lighting and the environment - Make the patient comfortable - Choose the examination sequence - Wash your hands in the presence of the patient - Describe your plans for the patient - Draped the patient. -

Comparability Ratios for ICDA-8 and ICD-9-CM
Comparability of Diagnostic Data Coded by the 8th and 9th Revisions of the International Classification of Diseases This report describes how the changes in the classification system used to code diagnoses reported in the National Hospital Discharge and the National Ambulatory Medical Care Surveys for utilization occurring in 1979 and later affect the comparability with similar data collected for utilization from 1968 through 1978. Comparability ratios are developed by coding the data using both coding classification revisions and dividing the national estimate based on one revision by the estimate based on the other revision. The comparability ratios can be used to estimate what the values published using one revision of the classification system would have been had coding been conducted according to the other revision. Data Evaluation and Methods Research Series 2, No. 104 DHHS Publication No. (PHS) 87–1 378 U.S. Department of Health and Human Services Public Health Service National Center for Health Statistics Hyattsville, Md. July 1987 Copyright information All material appearing in this report is in the public domain and may be reproduced or copied without permission; citation as to source, however, is appreciated. Suggasted citation National Center for Health Statistics, B. C. Duggar and W. F. Lew!s: Comparability of diagnostic data coded by the 8th and 9th revisions of the International Classification of Diseases. Vita/ and Heakh Statistics. Series 2, No. 104. DHHS Pub. No. (PHS) 87-1378. Public Health Service, Washington. U.S. Government Printing Office, July 1987 Library of Congrass Cataloging-in-Publication Data Duggar, 8enjamln C. Comparability of diagnostic data coded by the 8th and 9th revisions of the International Classification of Diseases. -

State of Illinois Eye Examination Report
State of Illinois State of Illinois Illinois Department of Public Health Eye Examination Report Illinois law requires that proof of an eye examination by an optometrist or physician (such as an ophthalmologist) who pro - vides eye examinations be submitted to the school no later than October 15 of the year the child is first enrolled or as re - quired by the school for other children. The examination must be completed within one year prior to the first day of the school year the child enters the Illinois school system for the first time. The parent of any child who is unable to obtain an examination must submit a waiver form to the school. Student Name _____________________________________________________________________________________ (Last) (First) (Middle Initial) Birth Date _________________ Gender ______ Grade ______ (Month/Day/Year) Parent or Guardian _________________________________________________________________________________ (Last) (First) Phone ___________________________ (Area Code) Address __________________________________________________________________________________________ (Number) (Street) (City) (ZIP Code) County _______________________________________ To Be Completed By Examining Doctor Case History Date of exam ________________ Ocular history: q Normal or Positive for ___________________________________________________________ Medical history: q Normal or Positive for ___________________________________________________________ Drug allergies: q NKDA or Allergic to ____________________________________________________________ -

Contemporary Coding Concepts
Contemporary Coding Concepts - 2011 John A. McGreal Jr., O.D. Missouri Eye Associates McGreal Educational Institute Excellence in Optometric Education John A. McGreal Jr., O.D. McGreal Educational Institute Missouri Eye Associates 11710 Old Ballas Rd. St. Louis, MO. 63141 314.569.2020 314.569.1596 FAX [email protected] JAM 2011 Medicare E/M Guidelines Compliance – How To Document the Medical Record – How To Select an E/M Codes, eye codes, “S” codes – How To Evaluate your Fees – How To Effectively Co-manage Surgical Cases – How To Increase Revenues – How To Survive an Audit – How To Implement a Compliance Plan JAM Other Medicare Benefits Changes Deductible (Medicare Part B) – Will increase to $162 in 2011; thereafter increase by annual percentage increase in Part B expenditures Deductible (Medicare Part A hospital inpatient) – Will be $1,132. Preventive benefits – beginning in 2005, all newly enrolled beneficiaries will be eligible for initial routine physical examinations, ECG, cardiovascular blood screening tests, education, counseling and referral for other preventive services and chronic care programs – 2011 wellness visits once yearly with PCP JAM 2006 New ICD-9 Codes Code first diabetes (250.5) 362.03 Nonproliferative diabetic retinopathy NOS 362.04 Mild nonproliferative diabetic retinopathy 362.05 Moderate nonproliferative diabetic retinopathy 362.06 Severe nonproliferative diabetic retinopathy 362.07 Diabetic macular edema – Must report with ICD code for diabetic retinopathy 362.01 = background diabetic retinopathy -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular -

State of Maine EYE EXAMINATION FORM
State of Maine Bureau of Motor Vehicles EYE EXAMINATION FORM THIS SECTION TO BE COMPLETED BY DRIVER (please print) Name ____________________________________ Date of Birth ___________________________ Address __________________________________ Driver’s License Number __________________ _________________________________________ Telephone _____________________________ TO BE COMPLETED BY LICENSED VISION EXAMINER Based on your examination of this patient and according to Functional Ability Profile rules (FAP). VISUAL ACUITY Without Correction With Correction Right eye 20/_____ 20/_____ Left eye 20/_____ 20/_____ Telescopic or bioptic lenses are not permitted for the visual acuity tests above. VISUAL FIELDS If visual field is less than 50° to left & 50° to right of fixation, or if less than 120° total, see FAP guidelines. Left of point of fixation __________ Right of point of fixation __________ TOTAL Degrees __________ Fresnel paste on prism lenses are not permitted for the visual field tests above. OCULAR MOTILITY Is there definite ocular motility that is likely to produce diplopia or other safety hazard? Yes No Please provide FAP profile level & treatment required to correct diplopia____________________________ OTHER EYE CONDITIONS (please indicate those that apply) Driver has possible progressive visual defect Reexamination recommended in(specify)____________ Patient uses bioptic telescopic lenses for driving Patient has hemianopsia LICENSE RESTRICTIONS Corrective lenses – Corrected visual acuity of 20/100 or better. Daylight only driving -

HEDIS Refresher- Eye Exam Medical Record Documentation Webinar
HEDIS Refresher- Eye Exam Medical Record Documentation Webinar Presented by: Martha Layne, RN, MHA HEDIS Clinical Lead, PHC Webinar Instructions Eureka | Fairfield | Redding | Santa Rosa Webinar Instructions • All participants have been muted to eliminate any possible noise interference/distraction. • If you have a question or would like to share your comments during the webinar, please type your question in the “question” box or click on the “raised hand” icon. Eureka | Fairfield | Redding | Santa Rosa Objectives • Overview of HEDIS & Partnership Healthplan • CDC- Eye Exam indicator Pathology of Diabetic Retinopathy Importance of eye exams for members dx w/Diabetes Importance of eye exam results being communicated w/PCP Documentation to provide from the eye exam for HEDIS • Contacts & Resources • Q&A Eureka | Fairfield | Redding | Santa Rosa Intro to PHC Southeast: Solano, Yolo, Napa Southwest: Sonoma, Marin, Mendocino, Lake Northeast: Lassen, Modoc, Siskiyou, Trinity, Shasta Northwest: Humboldt, Del Norte Eureka | Fairfield | Redding | Santa Rosa Overview • What is HEDIS? • Healthcare Effectiveness Data and Information Set • Why is HEDIS Important? • Evaluates clinical quality in a standardized way • Identifies opportunities for improvement • Regional-level performance is publicly reported • Regional-level reporting is required by the State • HEDIS/CAHPS equates to 50% of NCQA Accreditation Score Eureka | Fairfield | Redding | Santa Rosa Overview Measure Reporting Methodology: • Administrative Measures • Measures the entire eligible -

An Overview of BLENREP and Corneal Adverse Reactions for Eye
An Overview of BLENREP and Corneal Adverse Reactions for Eye Care Professionals Patients with relapsed or refractory multiple myeloma Table of Contents prescribed BLENREP (belantamab mafodotin) require an eye exam at baseline, before the subsequent 3 treatment cycles, and as clinically indicated whilst on treatment.1 Understanding a Patient With Relapsed/Refractory Multiple Myeloma .........4 Adverse reactions (ARs) have been reported with BLENREP, including corneal events, Overview of BLENREP ....................................................6 during clinical trials. Patients may be referred Efficacy of BLENREP in Patients With Relapsed/Refractory Multiple Myeloma ..8 to you by a haematologist/oncologist or Corneal ARs Observed in the DREAMM-2 (Study 205678) Clinical Trial .......10 may see you directly for their eye exams. MONITOR, MINIMISE, MODIFY: The 3 Ms of Corneal AR Management ........12 This guide is intended to provide you Dose Modifications for Corneal ARs ......................................18 an overview of why eye exams are required and what corneal ARs could Frequently Asked Questions .............................................20 potentially occur with BLENREP. References ............................................................23 It is important to communicate these findings to the haematology/ oncology care team, as findings of the eye exam(s) may impact the patient’s treatment. Corneal ARs are not the only risks associated with BLENREP.1 2 3 Understanding a Patient With Relapsed/Refractory Multiple Myeloma Multiple -

Booklet for High Myopia
Facts You Need to Know About CustomVue™ Laser Assisted In-Situ Keratomileusis (LASIK) Laser Treatment Patient Information Booklet For High Myopia (Nearsightedness) from -6 D to -11 D MRSE with up to -3 D Astigmatism Please read this entire booklet. Discuss its contents with your doctor so that all your questions are answered to your satisfaction. Ask any questions you may have before you agree to the surgery. VISX, Incorporated 3400 Central Expressway Santa Clara, CA 95051-0703 U.S.A. Tel: 408.733.2020 © Copyright 2005 by VISX, Incorporated All Rights Reserved VISX®, VisionKey®, ActiveTrak®, WaveScan®, WaveScan WaveFront®, and WavePrint® are registered trademarks of VISX, Incorporated. CustomVue™ and STAR S4™ are trademarks of VISX, Incorporated. Accutane® is a registered trademark of Hoffmann-La Roche Inc. Cordarone® is a registered trademark of Sanofi-Synthelabo, Inc. Imitrex® is a registered trademark of GlaxoSmithKline. No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from VISX, Incorporated. Table of Contents Introduction . 1 How Refractive (Wavefront) Errors Affect Your Vision . 2 The VISX STAR S4™ Excimer Laser . 4 How the CustomVue™ LASIK Procedure Works . 5 The VISX WaveScan WaveFront® System . 6 BENEFITS. 6 Clinical Study to Evaluate Benefits . 6 Study Patient Demographics . 7 Visual Acuity Without Glasses After Treatment . 8 Visual Acuity Without Glasses After Treatment and With Glasses Before Treatment . 9 Patient Quality of Vision Comparison . 10 RISKS . 11 CONTRAINDICATIONS — When Can’t You Have LASIK? . 12 What Warnings and Other Information Do You Need to Know About? .