Spatial Variability of Coral Bleaching in Palau During a Regional Thermal Stress Event in 2010
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of Fish and Invertebrates Listed in the CITES Appendices
JOINTS NATURE \=^ CONSERVATION COMMITTEE Checklist of fish and mvertebrates Usted in the CITES appendices JNCC REPORT (SSN0963-«OStl JOINT NATURE CONSERVATION COMMITTEE Report distribution Report Number: No. 238 Contract Number/JNCC project number: F7 1-12-332 Date received: 9 June 1995 Report tide: Checklist of fish and invertebrates listed in the CITES appendices Contract tide: Revised Checklists of CITES species database Contractor: World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge, CB3 ODL Comments: A further fish and invertebrate edition in the Checklist series begun by NCC in 1979, revised and brought up to date with current CITES listings Restrictions: Distribution: JNCC report collection 2 copies Nature Conservancy Council for England, HQ, Library 1 copy Scottish Natural Heritage, HQ, Library 1 copy Countryside Council for Wales, HQ, Library 1 copy A T Smail, Copyright Libraries Agent, 100 Euston Road, London, NWl 2HQ 5 copies British Library, Legal Deposit Office, Boston Spa, Wetherby, West Yorkshire, LS23 7BQ 1 copy Chadwick-Healey Ltd, Cambridge Place, Cambridge, CB2 INR 1 copy BIOSIS UK, Garforth House, 54 Michlegate, York, YOl ILF 1 copy CITES Management and Scientific Authorities of EC Member States total 30 copies CITES Authorities, UK Dependencies total 13 copies CITES Secretariat 5 copies CITES Animals Committee chairman 1 copy European Commission DG Xl/D/2 1 copy World Conservation Monitoring Centre 20 copies TRAFFIC International 5 copies Animal Quarantine Station, Heathrow 1 copy Department of the Environment (GWD) 5 copies Foreign & Commonwealth Office (ESED) 1 copy HM Customs & Excise 3 copies M Bradley Taylor (ACPO) 1 copy ^\(\\ Joint Nature Conservation Committee Report No. -

Species Diversity of Mushroom Corals (Family Fungiidae) in the Inner Gulf of Thailand
The Natural History Journal of Chulalongkorn University 2(2): 47-49, August 2002 ©2002 by Chulalongkorn University Species Diversity of Mushroom Corals (Family Fungiidae) in the Inner Gulf of Thailand LALITA PUTCHIM, SUCHANA CHAVANICH * AND VORANOP VIYAKARN Department of Marine Science, Faculty of Science, Chulalongkorn University, Bangkok 10330, THAILAND Mushroom coral (Family Fungiidae) is one islands (Fig. 1, Table 1). These species included of the most conspicuous groups in the tropical Ctenactis crassa (Dana, 1846), C. echinata Indo-Pacific reefs. These corals usually aggre- (Pallas, 1766), Fungia fungites (Linnaeus, gate in large clumps that are able to create the 1758), Lithophyllon undulatum Rehberg, 1892, reef formation (Pichon, 1974; Littler et al., Podabacia crustacea (Pallas, 1766), and Poly- 1997). In the tropical Indo-Pacific region, 41 phyllia talpina (Lamarck, 1801) (Fig. 1). Each species of fungiid corals have been found (Hoek- study site had four species of fungiids, but only sema, 1989). However, their biogeography is two species overlapped between the two sites. yet still unclear. In the Gulf of Thailand, little F. fungites, C. echinata, L. undulatum, and P. is known about the species diversity of fungiids crustacea were found at Ko Kham while F. and their distribution. Seven species were fungites, C. crassa, C. echinata, and P. talpina recorded by field survey at the Sichang Islands, were found at Ko Khram. Chon Buri Province (Sakai et al., 1986; Sara- From observations at Ko Khram and Ko sas, 1994), and 14 species were found in the Kham, it is interesting to note that more than coral collections at the institutes and museums 50% of live corals found in the study areas around the country (Jiravat, 1985). -

Growth and Population Dynamic Model of the Reef Coral Fungia Granulosa Klunzinger, 1879 at Eilat, Northern Red Sea
Journal of Experimental Marine Biology and Ecology View metadata, citation and similar papers at core.ac.uk L brought to you by CORE 249 (2000) 199±218 www.elsevier.nl/locate/jembe provided by Almae Matris Studiorum Campus Growth and population dynamic model of the reef coral Fungia granulosa Klunzinger, 1879 at Eilat, northern Red Sea Nanette E. Chadwick-Furmana,b,* , Stefano Goffredo c , Yossi Loya d aInteruniversity Institute for Marine Science, P.O. Box 469, Eilat, Israel bFaculty of Life Sciences, Bar Ilan University, Ramat Gan, Israel cDepartment of Evolutionary and Experimental Biology, University of Bologna, via Selmi 3, I-40126 Bologna, Italy dDepartment of Zoology, The George S. Wise Faculty of Life Sciences, and the Porter Super-Center for Ecological and Environmental Studies, Tel Aviv University, Tel Aviv, Israel Received 18 August 1999; received in revised form 10 February 2000; accepted 9 March 2000 Abstract The lack of population dynamic information for most species of stony corals is due in part to their complicated life histories that may include ®ssion, fusion and partial mortality of colonies, leading to an uncoupling of coral age and size. However, some reef-building corals may produce compact upright or free-living individuals in which the above processes rarely occur, or are clearly detectable. In some of these corals, individual age may be determined from size, and standard growth and population dynamic models may be applied to gain an accurate picture of their life history. We measured long-term growth rates (up to 2.5 years) of individuals of the free-living mushroom coral Fungia granulosa Klunzinger, 1879 at Eilat, northern Red Sea, and determined the size structure of a population on the shallow reef slope. -

Volume 2. Animals
AC20 Doc. 8.5 Annex (English only/Seulement en anglais/Únicamente en inglés) REVIEW OF SIGNIFICANT TRADE ANALYSIS OF TRADE TRENDS WITH NOTES ON THE CONSERVATION STATUS OF SELECTED SPECIES Volume 2. Animals Prepared for the CITES Animals Committee, CITES Secretariat by the United Nations Environment Programme World Conservation Monitoring Centre JANUARY 2004 AC20 Doc. 8.5 – p. 3 Prepared and produced by: UNEP World Conservation Monitoring Centre, Cambridge, UK UNEP WORLD CONSERVATION MONITORING CENTRE (UNEP-WCMC) www.unep-wcmc.org The UNEP World Conservation Monitoring Centre is the biodiversity assessment and policy implementation arm of the United Nations Environment Programme, the world’s foremost intergovernmental environmental organisation. UNEP-WCMC aims to help decision-makers recognise the value of biodiversity to people everywhere, and to apply this knowledge to all that they do. The Centre’s challenge is to transform complex data into policy-relevant information, to build tools and systems for analysis and integration, and to support the needs of nations and the international community as they engage in joint programmes of action. UNEP-WCMC provides objective, scientifically rigorous products and services that include ecosystem assessments, support for implementation of environmental agreements, regional and global biodiversity information, research on threats and impacts, and development of future scenarios for the living world. Prepared for: The CITES Secretariat, Geneva A contribution to UNEP - The United Nations Environment Programme Printed by: UNEP World Conservation Monitoring Centre 219 Huntingdon Road, Cambridge CB3 0DL, UK © Copyright: UNEP World Conservation Monitoring Centre/CITES Secretariat The contents of this report do not necessarily reflect the views or policies of UNEP or contributory organisations. -
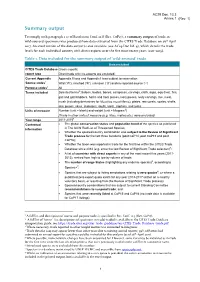
Summary Output
AC29 Doc. 13.3 Annex 1 Summary output To comply with paragraph 1 a) of Resolution Conf. 12.8 (Rev. CoP17), a summary output of trade in wild-sourced specimens was produced from data extracted from the CITES Trade Database on 26th April 2017. An excel version of the data output is also available (see AC29 Doc Inf. 4), which details the trade levels for each individual country with direct exports over the five most recent years (2011-2015). Table 1. Data included for the summary output of ‘wild-sourced’ trade Data included CITES Trade Database Gross exports; report type Direct trade only (re-exports are excluded) Current Appendix Appendix II taxa and Appendix I taxa subject to reservation Source codes1 Wild (‘W’), ranched (‘R’), unknown (‘U’) and no reported source (‘-’) Purpose codes1 All Terms included Selected terms2: baleen, bodies, bones, carapaces, carvings, cloth, eggs, egg (live), fins, gall and gall bladders, horns and horn pieces, ivory pieces, ivory carvings, live, meat, musk (including derivatives for Moschus moschiferus), plates, raw corals, scales, shells, skin pieces, skins, skeletons, skulls, teeth, trophies, and tusks. Units of measure Number (unit = blank) and weight (unit = kilogram3) [Trade in other units of measure (e.g. litres, metres etc.) were excluded] Year range 2011-20154 Contextual The global conservation status and population trend of the species as published information in The IUCN Red List of Threatened Species; Whether the species/country combination was subject to the Review of Significant Trade process for the last three iterations (post CoP14, post CoP15 and post CoP16); Whether the taxon was reported in trade for the first time within the CITES Trade Database since 2012 (e.g. -

Conservation of Reef Corals in the South China Sea Based on Species and Evolutionary Diversity
Biodivers Conserv DOI 10.1007/s10531-016-1052-7 ORIGINAL PAPER Conservation of reef corals in the South China Sea based on species and evolutionary diversity 1 2 3 Danwei Huang • Bert W. Hoeksema • Yang Amri Affendi • 4 5,6 7,8 Put O. Ang • Chaolun A. Chen • Hui Huang • 9 10 David J. W. Lane • Wilfredo Y. Licuanan • 11 12 13 Ouk Vibol • Si Tuan Vo • Thamasak Yeemin • Loke Ming Chou1 Received: 7 August 2015 / Revised: 18 January 2016 / Accepted: 21 January 2016 Ó Springer Science+Business Media Dordrecht 2016 Abstract The South China Sea in the Central Indo-Pacific is a large semi-enclosed marine region that supports an extraordinary diversity of coral reef organisms (including stony corals), which varies spatially across the region. While one-third of the world’s reef corals are known to face heightened extinction risk from global climate and local impacts, prospects for the coral fauna in the South China Sea region amidst these threats remain poorly understood. In this study, we analyse coral species richness, rarity, and phylogenetic Communicated by Dirk Sven Schmeller. Electronic supplementary material The online version of this article (doi:10.1007/s10531-016-1052-7) contains supplementary material, which is available to authorized users. & Danwei Huang [email protected] 1 Department of Biological Sciences and Tropical Marine Science Institute, National University of Singapore, Singapore 117543, Singapore 2 Naturalis Biodiversity Center, PO Box 9517, 2300 RA Leiden, The Netherlands 3 Institute of Biological Sciences, Faculty of -

Abundance and Distribution of Mushroom Corals
BULLETIN OF MARINE SCIENCE, 66(1): 241–254, 2000 CORAL REEF PAPER View metadata, citationABUNDANCE and similar papers AND at core.ac.uk DISTRIBUTION OF MUSHROOM CORALSbrought to you by CORE (SCLERACTINIA: FUNGIIDAE) ON A CORAL REEF ATprovided EILAT, by Almae Matris Studiorum Campus NORTHERN RED SEA S. Goffredo and N. E. Chadwick-Furman ABSTRACT Mushroom corals (Scleractinia: Fungiidae) are important components of Indo-Pacific coral reefs, yet little is known about their patterns of abundance and distribution in the Red Sea. On a fringing reef at Eilat, northern Red Sea, mushroom corals were found to be common on the reef flat and shallow slope at <10 m depth, where they occurred at densi- ties of up to 15 individuals m−2, but were rare on the mid to deep reef slope at 10–50 m depth. Eleven species were observed, a 27% increase in the recorded species diversity of this coral family at Eilat. Individuals of two species were limited to the reef flat and shallow reef slope, and members of five other species also occurred mainly on the shal- low slope, but had wide depth ranges. Individuals of an additional four mushroom coral species were found mainly on the lower reef slope at low densities. In shallow water, most fungiids occurred in shaded reef habitats such as caves or holes, while at >20 m depth, they mainly occupied open, unshaded habitats. This study documents differences in habi- tat use between species of mushroom corals on a fringing reef, and substantial migration by the adult free-living polyps of some species out of shaded reef habitats, down the reef slope, and onto soft substratum. -

A Molecularly Based Phylogeny Reconstruction of Mushroom Corals
Contributions to Zoology, 80 (2) 107-132 (2011) A molecularly based phylogeny reconstruction of mushroom corals (Scleractinia: Fungiidae) with taxonomic consequences and evolutionary implications for life history traits Arjan Gittenberger1, 2, Bastian T. Reijnen1, Bert W. Hoeksema1, 3 1 Department of Marine Zoology, Netherlands Centre for Biodiversity Naturalis, PO Box 9517, 2300 RA Leiden, The Netherlands 2 Institute for Environmental Sciences and Institute for Biology, Leiden University, PO Box 9516, 2300 RA Leiden, The Netherlands 3 E-mail: [email protected] Key words: COI, evolutionary history, ITS 1 & 2, maximum coral size, polystomatism, reproduction strategy Abstract Sequence alignment and phylogenetic analyses ............. 112 Evolution of life history traits ............................................. 113 The phylogenetic relationships of the Fungiidae, a family of pre- Results ............................................................................................. 113 dominantly free-living, zooxanthellate, reef corals, were studied Molecular phylogeny reconstructions .............................. 113 by sequencing a part of the mitochondrial Cytochrome Oxidase Evolutionary trends in morphology and life I (COI) and the complete ribosomal Internal Transcribed Spacers history traits ............................................................................. 114 (ITS) I & II of specimens from various locations in the Indo- Discussion ..................................................................................... -

Milleporidae, Stylasteridae, Scleractinia) in the National Museum of Natural History, Smithsonian Institution
Catalog of the Type Specimens of Stony Corals (Milleporidae, Stylasteridae, Scleractinia) in the National Museum of Natural History, Smithsonian Institution STEPHEN D. CAIRNS W9\ I SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 514 SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to the Marine Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Folklife Studies Smithsonian Studies in Air and Space Smithsonian Studies in History and Technology In these series, the Institution publishes small papers and full-scale monographs that report the research and collections of its various museums and bureaux or of professional colleagues in the world of science and scholarship. The publications are distributed by mailing lists to libraries, universities, and similar institutions throughout the world. Papers or monographs submitted for series publication are received by the Smithsonian Institution Press, subject to its own review for format and style, only through departments of the various Smithsonian museums or bureaux, where the manuscripts are given substantive review. -

The Reef Corals Lithactinia and Polyphyllia (Anthozoa, Scleractinia, Fungiidae): a Study of Morphological, Geographical, and Statistical Differences!
Pacific Science (1984), vol. 38, no. 1 © 1984 by the University of Hawaii Press. All rights reserved The Reef Corals Lithactinia and Polyphyllia (Anthozoa, Scleractinia, Fungiidae): A Study of Morphological, Geographical, and Statistical Differences! AUSTIN E. LAMBERTS2 ABSTRACT: The taxonomy of the Incl:o-Pacific reef corals Lithactinia and Polyphyllia is analyzed. They differ morphologically in that Lithactinia has one founder calice and no significant secondaries and has a lighter construction. A study ofbase area to weight ratios shows a significant difference, P < .001. They have a mutually exclusive geographical distribution. These data suggest that each is a valid genus. THE SCLERACTINIAN CORAL GENUS Polyphyllia surge of collections of marine specimens. is represented in most major coral collections Shells prized for beauty, rarity, and exotic and is well known. It is widespread in the origin fetched high prices and for a number of Indo-Pacific tropics, often rare, seldom years trading was brisk. Many important common, and never abundant. Because these national collections wery begun at that time. are free-living colonies found in shallow Naturalists had a field day publishing descrip waters, the coralla were easily collected and tions of new finds in richly illustrated books. transported and so are represented in many Many of these books were oriented to mala early descriptive works. Rumphius' (1750) cology but reef corals were at times included. specimen Fungus saxeus oblongus may have Thus, Seba in 1758 illustrated the modern been of this genus, and Seba's (1758) Marine genera Fungia, Herpolitha, apd Polyphyllia, taupe certainly was, although it was not men and Houttuyn in 1772 prodl.lced a richly il tioned by Linnaeus (1758), whose coral speci lustrated volume using the Linnean system in mens were largely from the Caribbean. -

Recent Changes in Scleractinian Coral Nomenclature and Classification
Recent changes in Scleractinian coral nomenclature and classification. (A practical guide for coral and reef ecologists) Michel Pichon Adjunct Professor, James Cook University Australia Honorary Associate, Museum of Tropical Queensland, Townsville The following document provides information on changes which were introduced recently in the classification and nomenclature of scleractinian corals. Such changes stem to a large extent from the research activity and the syntheses carried out by the members of the international “Scleractinian Systematics Working Group” (SSWG) over the last few years. They are the result of a multi-pronged approach combining the information provided by the implementation of relatively new techniques, which have only recently been applied to scleractinian coral taxonomy. Such new tools include, inter alia, morphometrics, microstructural analyses, anatomy of soft parts and molecular genetics. The changes thus made to the nomenclature and classification of scleractinian corals underlie a move towards a classification reflecting more and more the phylogeny of various taxa, and as a result may place side by side morphologically very dissimilar species. Furthermore, such a move will require a re-definition of numerous genera (and of some families, which from a practical viewpoint is less critical) in accordance with the rules set out in the International Code of Zoological Nomenclature. Such a work is still to be done and a number of genera remain to this day without an adequate revised diagnostic morphological characterization. Several important studies are still in progress and one may expect additional changes in the near future. The present document, therefore, does not pretend to represent a complete revision of scleractinian coral taxonomy. -

Feasibility Study on the Protection and Management of the Barren Isles Ecosystem, Madagascar
Garth Cripps Feasibility study on the protection and management of the Barren Isles ecosystem, Madagascar 309A/B Aberdeen Studios, 22-24 Highbury Grove, London N5 2EA, UK. [email protected] Tel: +44 (0)20 7359 1287 Fax: +44 (0)800 066 4032 © WWF 2011 . Copyright in this publication and in all text, data and images contained herein, except as otherwise indicated, rests with WWF. Keywords: Barren Isles, coral reefs, feasibility study, Madagascar, marine protected area. Acknowledgements: Financial support for this study was provided by WWF and the Natural Museum of Geneva through the: "Réseau interdisciplinaire pour une gestion durable de la biodiversité marine: diagnostic environnemental et social autour des tortues marines dans le sud-ouest de l’Océan Indien." Recommended citation: Cripps, G. 2010. Feasibility study on the protection and management of the Barren Isles ecosystem, Madagascar. Blue Ventures Conservation Report (2009), for WWF and the "Réseau interdisciplinaire pour une gestion durable de la biodiversité marine: diagnostic environnemental et social autour des tortues marines dans le sud-ouest de l’Océan Indien ". 272 pp Table of Contents List of figures ............................................................................................................................................................7 List of tables .............................................................................................................................................................9 Abbreviations and Acronyms .................................................................................................................................12