Description and Validation of the Simple, Efficient, Dynamic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hot Age Or Ice Age TABLE of CONTENTS
Imprint: Publisher: TerraFuture AG (i.Gr.) Hauptstr. 193 50169 Kerpen Germany Author: Wolf-Walter Stinnes (M.Sc.Phys.) Internet: www.TerraFuture.ag E-Mail: [email protected] Copyright ©2019 TerraFuture AG (i.Gr.) All rights reserved, in particular the right of duplication, distribution and/or translation. No part of the work may be reproduced in any form (including photocopying, microfilm or any other process) or stored, processed, duplicated or distributed using electronic or mechanical methods without the written consent of TerraFuture AG (i.Gr.). 2 Hot Age or Ice Age TABLE OF CONTENTS A. Author �������������������������������������������������������������������������������5 B. What are the Real Problems of Mankind ������������������������6 C. Why and how does the Earth´s Atmosphere warm up? ���������������������������������������������������������������������������8 1. Visible Solar Radiation �������������������������������������������������9 2. Invisible Solar or Space Radiation, infrared (IR) or ultraviolet (UV) ��������������������������������������������������������10 3. Heat Conduction from the inner Earth to its Surface: . 11 4. Heat Transfer from the Earth´s hot surface into the atmosphere by heat conduction ��������������������������� 11 5. Heat transfer into the atmosphere by convection ������� 11 D. The Role of Greenhouse Gases ....................................12 1. The Greenhouse Gas CO2: ����������������������������������������12 2. CO2 versus Water Vapor: �������������������������������������������12 3. The pretension -
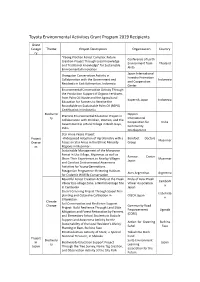
List of Previous Grant Projects
Toyota Environmental Activities Grant Program 2019 Recipients Grant Catego Theme Project Description Organization Country ry "Kaeng Krachan Forest Complex: Future Conference of Earth Creation Project Through Local Knowledge Environment from Thailand and Traditional Knowledge" for Sustainable Akita Environmental Innovation Japan International Orangutan Conservation Activity in Forestry Promotion Collaboration with the Government and Indonesia and Cooperation Residents in East Kalimantan, Indonesia Center Environmental Conservation Activity Through the Production Support of Organic Fertilizers from Palm Oil Waste and the Agricultural Kopernik Japan Indonesia Education for Farmers to Receive the Roundtable on Sustainable Palm Oil (RSPO) Certification in Indonesia Biodiversi Nippon Practical Environmental Education Project in ty International Collaboration with Children, Women, and the Cooperation for India Government in a Rural Village in Bodh Gaya, Community India Development Star Anise Peace Project Project -Widespread Adoption of Agroforestry with a Barefoot Doctors Myanmar Overse Focus on Star Anise in the Ethnic Minority Group as Regions in Myanmar- Sustainable Management of the Mangrove Forest in Uto Village, Myanmar, as well as Ramsar Center Share Their Experiences to Nearby Villages Myanmar Japan and Conduct Environmental Awareness Activities for Young Generations Patagonian Programme: Restoring Habitats Aves Argentinas Argentina for Endemic Wildlife Conservation Beautiful Forest Creation Activity at the Preah Pride of Asia: Preah -

Japan National Report Based on the United Nations Convention To
Japan National Report based on the United Nations Convention to Combat Desertification in those Countries Experiencing Serious Drought and/or Desertification, Particularly in Africa (UNCCD) April 2002 - 1 - TABLE OF CONTENTS I. EXECUTIVE SUMMARY 4 1. Placement of the Report 4 2. Efforts by both the international community and Japan regarding desertification 4 3. Japan’s various efforts under the United Nations Convention to Combat Desertification 5-7 II. AFRICA 1. Overview 8 A. Consultative process and partnership agreements 8 B. Measures taken to support the preparation and implementation of action programmes at all levels 9-10 2. Support for the UNCCD process 10 A. Financial support for various activities 11 3. Bilateral cooperation and other activities 11-31 A. Conservation of water resources 11-13 B. Forest conservation and re-afforestation 13- 15 C. Agricultural development 15-16 D. Capacity building and education 16-17 E. Study and research on desertification 17-19 F. Support for NGO activities 19-31 4. Contributions through international organizations 31-35 A. United Nations Environment Programme (UNEP) 32 B. Food and Agriculture Organization of the United Nations (FAO) 32 C. International Tropical Timber Organization (ITTO) 32 D. International Fund for Agricultural Development (IFAD) 32-33 E. United Nations Development Programme (UNDP) 33 F. World Meteorological Organization (WMO) 33 G.Consultative Group in International Agricultural Research(CGIAR) 33 H. International Bank for Reconstruction and Development(IBRD; the World Bank) 33 I. Global Environment Facility (GEF) 34 J. African Development Bank(AfDB) 34 K. Asian Development Bank (ADB) 34 L . World Food Programme(WFP) 34 M. -

Greening the Desert Greening the Desert
Greening The Desert Greening the desert project: 2-10 years plan in the frame of the 4 dimension And the involvement of Heliopolis university Greening The Desert New space for a new community What is the project goal? Our Goal Phase 1 Funding: 100% accomplished Installation 3 Pivots installed, solar panels under construction 150 fadan of desert land is reclaimed Phase 1: Summary of Funding Details( take out-replace 1 slide for phase 2- date and goal Kind of Contribution Amount Percentage CO2 €26,744.77 6.64% Loan €180,900.00 44.90% Donation €134,057.41 33.28% Tree Planting €61,169.69 15.18% Total €402,871.87 100.00% Vision plan for Greening the desert in the frame of the 4 dimensions Cultural Life 2 years plan 10 years plan SVG1: Educational Model SVG 1: Educational Model - Education program for the SEKEM Wahat community members is established- (Hu A holistic school in founde vision group of unfolding potential.Regina) - Core program is established and held every A holistic teacher training programme is implemen month (Hu vision group of unfolding potential .Martina) A Special needs education program is establishe - For employees: Maho el omia for illiterate people above the age of 15- (Sekem school.Start Date September 2020) A Vocational Training Center is established - Cultural Life 2 years plan 10 years plan SVG 2: University model SVG 2: University model - Internships in all different working fields - HU Desert Campus is founded (practical are offered for HU and international modules are hold in Wahat) students to participate in the -

Contraction of the Gobi Desert, 2000–2012
Remote Sens. 2015, 7, 1346-1358; doi:10.3390/rs70201346 OPEN ACCESS remote sensing ISSN 2072-4292 www.mdpi.com/journal/remotesensing Article Contraction of the Gobi Desert, 2000–2012 Troy Sternberg *, Henri Rueff and Nick Middleton School of Geography, University of Oxford, South Parks Road, Oxford OX1 3QY, UK; E-Mails: [email protected] (H.R.); [email protected] (N.M.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +44-186-5285-070. Academic Editors: Arnon Karnieli and Prasad S. Thenkabail Received: 5 September 2014 / Accepted: 21 January 2015 / Published: 26 January 2015 Abstract: Deserts are critical environments because they cover 41% of the world’s land surface and are home to 2 billion residents. As highly dynamic biomes desert expansion and contraction is influenced by climate and anthropogenic factors with variability being a key part of the desertification debate across dryland regions. Evaluating a major world desert, the Gobi in East Asia, with high resolution satellite data and the meteorologically-derived Aridity Index from 2000 to 2012 identified a recent contraction of the Gobi. The fluctuation in area, primarily driven by precipitation, is at odds with numerous reports of human-induced desertification in Mongolia and China. There are striking parallels between the vagueness in defining the Gobi and the imprecision and controversy surrounding the Sahara desert’s southern boundary in the 1980s and 1990s. Improved boundary definition has implications for understanding desert “greening” and “browning”, human action and land use, ecological productivity and changing climate parameters in the region. -

Land for Life CREATE WEALTH TRANSFORM LIVES Land for Life for Land
Land for Life CREATE WEALTH TRANSFORM LIVES Land for Life | CREATE WEALTH —TRANSFORM LIVES WEALTH CREATE CREATE WEALTH TRANSFORM LIVES As a mother is Allowing us to cultivate her soil Mother Earth gives her land to us for our own bidding We must, in turn, Return the favor Nurture Mother Earth, as she nurtured us Extract of poem, “Mother Earth,” by Yen Li Yeap (13 years old) © 2016 UNCCD and World Bank UNCCD Secretariat Langer Eugen, Platz der Vereinten Nationen 1 D-53113 Bonn, Germany Tel: +49-228 / 815-2800 Fax: +49-228 / 815-2898/99 Email: [email protected] World Bank 1818 H Street, NW Washington, DC 20433 USA Tel: (202) 473-1000 Fax: (202) 477-6391 Internet: www.worldbank.org E-mail: [email protected] All rights reserved. This publication is a product of the staff of UNCCD and World Bank. It does not necessarily reflect the views of the UNCCD or World Bank/TerrAfrica or the member governments they represent. The UNCCD and World Bank/TerrAfrica do not guarantee the accuracy of the data included in this work. The boundaries, colors, denominations, and other information shown on any map in this work do not imply any judgment on the part of the UNCCD and World Bank concerning the legal status of any territory or the endorsement or acceptance of such boundaries. RIGHTS AND PERMISSIONS The material in this publication is copyrighted. Copying and/or transmitting portions or all of this work without permission may be a violation of applicable law. The UNCCD and World Bank encourage dissemination of its work and will normally grant permission to reproduce portions of the work promptly. -

Research and Development to Improve Desert Environments And
OObservationbservation bbyy ssatelliteatellite Meteorological-Meteorological- andand Research and Development to environmental-observationenvironmental-observation AdvancedAdvanced predictionprediction Improve Desert Environments systemsystem ofof regionalregional Promoting rainfall waterwater cyclecycle and Create a Sustainable Rainfall Wastewater Atmospheric moisture radar Desert greening collection system treatment by deep-rooted Oasis Network system with seeding air-conditioning equipment Seawater desalination by solar energy 1 2 and biomass MAKOTO HARADA* RYOHJI OHBA* WATARU KAWAMURA*3 MASAHIKO NAGAI*4 As economic development and urbanization have expanded in the Middle East, so too has desertification. Secur- ing adequate water resources to support economic development and enhance urban environments is critical. Accord- ingly, adopting highly efficient energy-supply equipment and technologies for securing water resources, using water effectively, and implementing desert greening or reforesting projects are important. Mitsubishi Heavy Industries, Ltd. (MHI) aims to contribute to the stable and continuous economic development and environmental improvement of Middle Eastern countries and, in turn, to strengthen the energy security of Japan. Specifically, using its technolo- gies and products, MHI proposes a scenario for creating a sustainable "oasis." This paper introduces some of MHI's efforts to improve desert environments with the goal of creating such a sustainable desert oasis. academic organizations, MHI has undertaken various 1. Project Overview: Improving Desert Environments engineering studies related to global and regional cli- 1.1 Creating a sustainable oasis network mate change. Middle Eastern countries continue to enjoy remark- Such studies have revealed the possibility of green- able progress in economic development and urbanization. ing the so-called coastal deserts, many of which exist in However, desertification and a lack of water resources western coastal areas of continents. -

United Nations Convention to Combat Desertification (UNCCD)
UNITED NATIONS Distr. Convention to Combat GENERAL Desertification ICCD/CRIC(1)/3/Add.2 17 October 2002 ENGLISH ONLY COMMITTEE FOR THE REVIEW OF THE IMPLEMENTATION OF THE CONVENTION First session Rome, 11-22 November 2002 Item 3 (a) of the provisional agenda REVIEW OF THE IMPLEMENTATION OF THE CONVENTION, PURSUANT TO ARTICLE 22, PARAGRAPH 2(A) AND (B), AND ARTICLE 26 OF THE CONVENTION REVIEW OF REPORTS ON IMPLEMENTATION BY AFFECTED ASIAN COUNTRY PARTIES, INCLUDING ON THE PARTICIPATORY PROCESS, AND ON EXPERIENCE GAINED AND RESULTS ACHIEVED IN THE PREPARATION AND IMPLEMENTATION OF ACTION PROGRAMMES Addendum COMPILATION OF SUMMARIES OF REPORTS SUBMITTED BY ASIAN COUNTRY PARTIES1 Note by the secretariat 1. By its decision 1/COP.5, the Conference of the Parties (COP) decided to establish a committee for the review of the implementation of the Convention (CRIC). It decided also that the first session of the CRIC, to be held in November 2002, shall review updates to reports already available and/or new reports from all regions. 2. Furthermore, pursuant to decision 11/COP.1, the secretariat was requested to compile the summaries of reports submitted by affected country Parties and any group of affected country Parties that makes a joint communication, directly or through a competent subregional or regional organization. The same decision also defined the format and content of reports and required, in particular, a summary of the national report not to exceed six pages. 3. The present document contains the summaries of reports submitted by 33 Asian country Parties. The secretariat has also made these reports available on its Web site (http://www.unccd.int). -

Applying the Concept of “Energy Return on Investment” to Desert Greening of the Sahara/Sahel Using a Global Climate Model
Earth Syst. Dynam., 5, 43–53, 2014 Open Access www.earth-syst-dynam.net/5/43/2014/ Earth System doi:10.5194/esd-5-43-2014 © Author(s) 2014. CC Attribution 3.0 License. Dynamics Applying the concept of “energy return on investment” to desert greening of the Sahara/Sahel using a global climate model S. P. K. Bowring1, L. M. Miller2, L. Ganzeveld1, and A. Kleidon2 1Earth System Science Group, Wageningen University and Research Centre, Wageningen, the Netherlands 2Max Planck Institute for Biogeochemistry, Jena, Germany Correspondence to: S. P. K. Bowring ([email protected]) Received: 30 July 2013 – Published in Earth Syst. Dynam. Discuss.: 8 August 2013 Revised: 9 December 2013 – Accepted: 21 December 2013 – Published: 30 January 2014 Abstract. Altering the large-scale dynamics of the Earth 1 Introduction system through continual and deliberate human intervention now seems possible. In doing so, one should question the en- ergetic sustainability of such interventions. Here, from the Numerous human activities revolve around the capture and basis that a region might be unnaturally vegetated by em- use of energy. The potential now exists for humans to ap- ploying technological means, we apply the metric of “en- propriate additional energy (e.g., renewable technologies), ergy return on investment” (EROI) to benchmark the ener- which on a large scale may alter the underlying dynamics getic sustainability of such a scenario. We do this by apply- of Earth system processes (e.g., via land-use change). Yet ing EROI to a series of global climate model simulations energy on Earth is limited by the flow of incoming solar ra- where the entire Sahara/Sahel region is irrigated with in- diation, its “heat of formation”, gravitational attraction by ce- creased rates of desalinated water to produce biomass. -

No.191 November 1, 2013 Strategy to Develop Frontier Markets With
No. 191 November 1, 2013 Strategy to Develop Frontier Markets with Emphasis on Adaptation to Climate Change Junji KOIKE, Tokutaro HIRAMOTO and Takanori IZUMI NRI Papers No. 191 November 1, 2013 Strategy to Develop Frontier Markets with Emphasis on Adaptation to Climate Change Junji KOIKE, Tokutaro HIRAMOTO and Takanori IZUMI I Effects of Accelerating Climate Change and Frontier Markets for Private-Sector Enterprises in Developed Countries II Wave of Adaptation Business Spreads among Enterprises in Europe, the U.S. and Emerging Countries III Beginning of Adaptation Business by Japanese Companies IV Steps to Develop Frontier Markets with Attention Placed on Climate Change limate change not only affects the global environment, as seen in the changes in ecosystems C and poor harvests that are caused by temperature rise, changes in rainfall patterns that lead to unseasonable droughts and heavy rains, and spread of salt damage caused by rises in the sea level, but it also has a major impact on society and the economy. Any response to these challenges will require the increased use of not only public, but also private funds. For private-sector enterprises in developed countries, business opportunities presented by adap- tive measures in response to climate change are found extensively in various sectors. For business operated in emerging and developing countries, an approach that takes in not only consumer mar- kets but also local governments and local companies is essential to expanding the scale of business and stabilizing earnings. From this point of view, it is possible to say that adaptive measures in response to climate change will come to form a major market in the future. -

UAV-Supported Forest Regeneration: Current Trends, Challenges and Implications
remote sensing Review UAV-Supported Forest Regeneration: Current Trends, Challenges and Implications Midhun Mohan 1,2,*, Gabriella Richardson 2, Gopika Gopan 2, Matthew Mehdi Aghai 3, Shaurya Bajaj 2, G. A. Pabodha Galgamuwa 2,4 , Mikko Vastaranta 5 , Pavithra S. Pitumpe Arachchige 2 , Lot Amorós 6 , Ana Paula Dalla Corte 7, Sergio de-Miguel 8,9 , Rodrigo Vieira Leite 10 , Mahlatse Kganyago 11,12 , Eben North Broadbent 13 , Willie Doaemo 2,14,15 , Mohammed Abdullah Bin Shorab 16 and Adrian Cardil 8,9,17 1 Department of Geography, University of California—Berkeley, Berkeley, CA 94709, USA 2 United Nations Volunteering Program, Morobe Development Foundation, Lae 00411, Papua New Guinea; [email protected] (G.R.); [email protected] (G.G.); [email protected] (S.B.); [email protected] (G.A.P.G.); [email protected] (P.S.P.A.); [email protected] (W.D.) 3 DroneSeed Co., 1123 NW 51st Street, Seattle, WA 25198, USA; [email protected] 4 The Nature Conservancy, Maryland/DC Chapter, Cumberland, MD 21502, USA 5 School of Forest Sciences, University of Eastern Finland, 80101 Joensuu, Finland; mikko.vastaranta@uef.fi 6 Dronecoria, 03202 Elche, Spain; [email protected] 7 Department of Forest Engineering, Federal University of Paraná—UFPR, Curitiba 80210-170, PR, Brazil; [email protected] 8 Joint Research Unit CTFC—AGROTECNIO—CERCA, 25198 Solsona, Spain; [email protected] (S.d.-M.); [email protected] (A.C.) 9 Department of Crop and Forest Sciences, University of Lleida, 25003 Lleida, Spain 10 Department of Forest Engineering, Federal University of Viçosa, Viçosa 36570-900, MG, Brazil; Citation: Mohan, M.; Richardson, G.; [email protected] 11 Gopan, G.; Aghai, M.M.; Bajaj, S.; Earth Observation Directorate, South African National Space Agency, Pretoria 0001, South Africa; Galgamuwa, G.A.P.; Vastaranta, M.; [email protected] 12 School of Geography, Archaeology and Environmental Studies, University of the Witwatersrand, Arachchige, P.S.P.; Amorós, L.; Corte, Johannesburg 2050, South Africa A.P.D.; et al. -

Tree Plantation
TREE PLANTATION CDT: SK MD TOUFIK REGIMENTAL NO: WB/19/SDA/ 192-218 COLLAGE : SYAMSUNDAR COLLAGE 4 Bengal Bn NCC Burdwan Group HQ West Bengal & Sikkim Directorate Tree-planting is the process of transplanting tree seedlings, generally for forestry, land reclamation,or landscaping purpose. It differs from the transplantation of larger trees in arboriculture, and from the lower cost but slower and less reliable distribution of tree seeds. Because trees remove carbon dioxide from the air as they grow, tree planting can be used as a geoengineering technique to remove CO2 from the atmosphere. Desert greening projects are also motivated by improved biodiversity and reclamation of natural water systems, but also improved economic and social welfare due to an increased number of jobs in farming and forestry. Tree Plantation drives combat many environmental issues like deforestation, erosion of soil, desertification in semi-arid areas, global warming and hence enhancing the beauty and balance of the environment. Trees absorb harmful gases and emit oxygen resulting in increase in oxygen supply. On an average, a single tree emits 260 pounds of oxygen annually. Similarly, a fully-grown tree is sufficient for 18 human beings in one acre of land in one year stressing the importance of tree plantation for mankind. The type of tree planted may have great influence on the environmental outcomes. It is often much more profitable to outside interests to plant fast-growing species, such as eucalyptus, casuarina. To promote the growth of native ecosystems, many environmentalists advocate only indigenous Trees be planted. A practical solution is to plant tough, fast-growing native tree species which begin rebuilding the land.