E-Cigarettes: the Vapor This Time?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
FY 2020 Announcement.Pdf
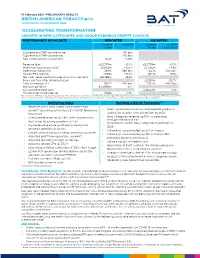
17 February 2021 –PRELIMINARY RESULTS BRITISH AMERICAN TOBACCO p.l.c. YEAR ENDED 31 DECEMBER 2020 ‘ACCELERATING TRANSFORMATION’ GROWTH IN NEW CATEGORIES AND GROUP EARNINGS DESPITE COVID-19 PERFORMANCE HIGHLIGHTS REPORTED ADJUSTED Current Vs 2019 Current Vs 2019 rates Rates (constant) Cigarette and THP volume share +30 bps Cigarette and THP value share +20 bps Non-Combustibles consumers1 13.5m +3.0m Revenue (£m) £25,776m -0.4% £25,776m +3.3% Profit from operations (£m) £9,962m +10.5% £11,365m +4.8% Operating margin (%) +38.6% +380 bps +44.1% +100 bps2 Diluted EPS (pence) 278.9p +12.0% 331.7p +5.5% Net cash generated from operating activities (£m) £9,786m +8.8% Free cash flow after dividends (£m) £2,550m +32.7% Cash conversion (%)2 98.2% -160 bps 103.0% +650 bps Borrowings3 (£m) £43,968m -3.1% Adjusted Net Debt (£m) £39,451m -5.3% Dividend per share (pence) 215.6p +2.5% The use of non-GAAP measures, including adjusting items and constant currencies, are further discussed on pages 48 to 53, with reconciliations from the most comparable IFRS measure provided. Note – 1. Internal estimate. 2. Movement in adjusted operating margin and operating cash conversion are provided at current rates. 3. Borrowings includes lease liabilities. Delivering today Building A Better TomorrowTM • Revenue, profit from operations and earnings • 1 growth* absorbing estimated 2.5% COVID-19 revenue 13.5m consumers of our non-combustible products , headwind adding 3m in 2020. On track to 50m by 2030 • New Categories revenue up 15%*, accelerating • Combustible revenue -

Vaping Lingo Dictionary
Vaping Lingo Dictionary High vaping rates among youth and young adults have introduced a plethora of new terms and keeping up with the latest lingo can be difficult. If you are wondering what the young people in your life are talking about, here is a list of some popular words, phrases, products and general language used to refer to vaping/e-cigarette use. POPULAR VAPE BRANDS & DEVICES Closed Pod System (uses disposable pods) JUUL BLU NJOY VUSE Vibe VUSE Alto Open/Refillable System Box Mod Suorin Drop Suorin Air NOVO Vape Juice Disposables BIDI Stick Cuvie (HQD) Puff Bars Posh Vape Stig POPULAR TERMS, PHRASES & SLANG USED BY YOUNG VAPERS The part that provides power to the heating element to warm Battery, Batt the e-liquid and produce vapor Blanks Empty cartridges a user can fill with the e-juice of their choice Cartridge, Cart A refillable vape juice container Used to recharge the e-cig battery once it has been depleted Charger Clone A knock-off of an original device that is typically less expensive A type of device that uses disposable pods containing e-liquid (typically ~200-500 puffs). The body of these devices can be recharged and the disposable pods can be replaced with new Closed Pod System compatible pods. What vapers call the vape mist that’s produced during vaping Clouds A Dab pen is used primarily for consuming THC concentrates and using the device is typically also referred to as “vaping.” These devices look and work much like other vape pens. Dab pen The “most prominent in a class of largely counterfeit brands, with common packaging that is easily available online and used by distributors to market THC-containing cartridges," according to a Centers for Disease Control and Prevention report on e- cigarette or vaping product use-associated lung injury. -

Low-Nitrosamine Cigarettes Go on Sale
Low-nitrosamine cigarettes go on sale ■ Star's Advance, which rette smoke. Advance cigarettes will also Star Scientific is the first tobacco com Dallas-Fort Worth. St.ax Scientific is t.aking contain reduced levels of such other toxins pany to sell low-nitrosamine cigarettes and a much different approach in marketing has lower levels of some as hydrogen cyanide, and their packaging the latest to market cigarettes that the Advance compared to RJR's marketing of will include detailed health warnings tak public might perceive as a safer smoking Eclipse. ' toxins, being test~marketed ing up the entire back of the cigarette alternative. Advance's two test markets are The c2mpany is making no health By Adrian Zawada packs. Richntond and Lexington, Ky. claims, admitting on its Advance cigarette JOURNAL REPORTER ''We have an obligation to make Advance R.J. Reynolds Tobacco Co. has invested packs that "there is not enough evidence St.ax Scientific Inc. began selling low available to adult tobacco consumers along more than $1 billion fu developing its available to know if Star's methods will nitrosamine cigarettes in selected Ken with infonnation about the comparable smokeless cigarette, Eclipse, and its failed actually lower your health risks." tucky and Virginia test markets yesterday toxic constituents of smoke," said Paul predecessor, Premier. RJR announced two weeks ago that com.; to attract smokers who want a cigarette Perito, the company's president and chair RJR's Eclipse heats tobacco instead of pany scientists found no reduction in the with less potential to cause cancer. man. "Star hopes that the start of its limit burning it, and the company says it might toxicity of low-nitrosamine tobacco when The cigarettes, called Advance, have 73 ed test market of Advance will serve as an present smokers with less risk of lung can exposing the smoke to animals and DNA percent less tobacco-specific ri.itrosamines incentive to the traditional tobacco indus cer, chronic bronchitis and emphysema. -

Sproule Et Al V. Santa Fe Natural Tobacco
Case 0:15-cv-62064-JAL Document 1 Entered on FLSD Docket 09/30/2015 Page 1 of 20 UNITED STATES DISTRICT COURT SOUTHERN DISTRICT OF FLORIDA JUSTIN SPROULE, individually and on behalf of all others similarly situated, No. Plaintiff, JURY TRIAL DEMANDED v. SANTA FE NATURAL TOBACCO COMPANY, INC., and REYNOLDS AMERICAN INC., Defendants. / CLASS ACTION COMPLAINT Plaintiff, Justin Sproule, individually, and on behalf of all others similarly situated in the United States, by and through the undersigned counsel, files this Class Action Complaint, and alleges against Defendants, Santa Fe Natural Tobacco Company, Inc. and Reynolds American Inc., as follows: INTRODUCTION 1. Defendants manufacture, market, and sell Natural American Spirit cigarettes (“American Spirits”). Defendants’ product labeling and advertising describes these cigarettes as “Natural,” “Additive Free,” “100% Additive Free,” “Organic,” and an “unadulterated tobacco product.”1 These terms are intended to suggest that American Spirits are healthier, safer, and present a lower risk of tobacco-related disease than other tobacco products. Defendants, however, have no competent or reliable scientific evidence to back their labeling and advertising claims. Defendants’ claims are patently deceptive, especially in today’s market, where these terms have a 1 https://www.sfntc.com/site/ourCompany/sfntc-story/ (last visited Aug. 28, 2015). Case 0:15-cv-62064-JAL Document 1 Entered on FLSD Docket 09/30/2015 Page 2 of 20 potent meaning for the health-and environmentally-conscious consumer. Moreover, as the FDA recently determined, American Spirits are in fact ‘adulterated.’ Using these deceptive terms, Defendants are able to successfully price American Spirits higher than other competitive cigarette brands. -

Cigarette Minimum Retail Price List
MASSACHUSETTS DEPARTMENT OF REVENUE FILING ENFORCEMENT BUREAU CIGARETTE AND TOBACCO EXCISE UNIT PRESUMPTIVE MINIMUM RETAIL PRICES EFFECTIVE July 26, 2021 The prices listed below are based on cigarettes delivered by the wholesaler and do not include the 6.25 percent sales tax. Brands of cigarettes held in current inventory may be sold at the new presumptive minimum prices for those brands. Changes and additions are bolded. Non-Chain Stores Chain Stores Retail Retail Brand (Alpha) Carton Pack Carton Pack 1839 $86.64 $8.66 $85.38 $8.54 1st Class $71.49 $7.15 $70.44 $7.04 Basic $122.21 $12.22 $120.41 $12.04 Benson & Hedges $136.55 $13.66 $134.54 $13.45 Benson & Hedges Green $115.28 $11.53 $113.59 $11.36 Benson & Hedges King (princess pk) $134.75 $13.48 $132.78 $13.28 Cambridge $124.78 $12.48 $122.94 $12.29 Camel All others $116.56 $11.66 $114.85 $11.49 Camel Regular - Non Filter $141.43 $14.14 $139.35 $13.94 Camel Turkish Blends $110.14 $11.01 $108.51 $10.85 Capri $141.43 $14.14 $139.35 $13.94 Carlton $141.43 $14.14 $139.35 $13.94 Checkers $71.54 $7.15 $70.49 $7.05 Chesterfield $96.53 $9.65 $95.10 $9.51 Commander $117.28 $11.73 $115.55 $11.56 Couture $72.23 $7.22 $71.16 $7.12 Crown $70.76 $7.08 $69.73 $6.97 Dave's $107.70 $10.77 $106.11 $10.61 Doral $127.10 $12.71 $125.23 $12.52 Dunhill $141.43 $14.14 $139.35 $13.94 Eagle 20's $88.31 $8.83 $87.01 $8.70 Eclipse $137.16 $13.72 $135.15 $13.52 Edgefield $73.41 $7.34 $72.34 $7.23 English Ovals $125.44 $12.54 $123.59 $12.36 Eve $109.30 $10.93 $107.70 $10.77 Export A $120.88 $12.09 $119.10 $11.91 -

BMW of North America, LLC NJ ""K"" Line America, Inc. VA 1199
The plan sponsors listed below have at least one application for the Retiree Drug Subsidy (RDS) program in an "Approved" status for a plan year ending in 2010 as of February 4, 2011. The state listed for each sponsor is the state provided by the sponsor on the application for the subsidy. This state may, or may not, be where the majority of the plan sponsor's retirees reside or where the plan sponsor is headquartered. This list will be updated periodically. Plan Plan Sponsor Business Name Sponsor State : BMW of North America, LLC NJ ""K"" Line America, Inc. VA 1199 SEIU Greater New York Benefit Fund NY 1199 SEIU National Benefit Fund NY 3M Company MN 4th District IBEW Health Fund WV A-C RETIREES' VOLUNTARY BENFITS PLAN WI A. DUDA & SONS, INC. FL A. SCHULMAN, INC OH A. T. Massey Coal Company, Inc. VA A&E Television Networks NY AAA EAST PENN PA AARP DC ABB Inc. CT Abbott Laboratories IL Abbott Pharmaceuticals PR Ltd. PR Acadia Parish School Board LA Accenture LLP IL Accuride Corporation IN ACF Industries LLC MO ACGME IL Acton Health Insurance Trust MA Actuant Corporation WI Adirondack Central School NY Administrative Office of the Pennsylvania Courts PA Adventist Risk Management MD Advisory Services OH AEGON USA, Inc. IA AFL-CIO Health and Welfare Trust DC AFSCME DC AFSCME Council 31 IL afscme d.c. 47 health & welfare fund PA AFSCME District Council 33 Health and Welfare Plan PA AFTRA Health Fund NY AGC FLAT GLASS NORTH AMERICA INC TN Page 1 AGC-IUOE Local 701 Health & Welfare Trust Fund WA AGCO Corporation GA Agilent Technologies, Inc. -

Case Study Industry: Tobacco
CASE STUDY INDUSTRY: TOBACCO CUSTOMER: R.J. Reynolds Tobacco Company LOCATION: Winston Salem, North Carolina BACKGROUND: R.J. Reynolds Tobacco Company is the second-largest tobacco company in the United States, offering products in all segments of the market and makes many of the nation’s best-selling cigarette brands, including: Camel, Pall Mall, Kool, Winston, Salem, Doral, and VUSE brand E-cigarette. SCOPE OF WORK: Armstrong International is responsible for the operation and maintenance of the utilities systems at the Tobaccoville and Whitaker Park Boiler and Process Services plants including all associated equipment to provide quality steam, condensate, chill water, compressed air, and water treatment to meet ISO, FDA, and process requirements of the R.J. Reynolds Tobacco Company manufacturing facilities. Armstrong provides one site manager, two operation and maintenance managers as well as 35 operation and maintenance support employees, which includes 18 utility plant operators, one water treatment specialist, four operations and maintenance lead technicians, 11 HVAC and instrumentation technicians, and four mechanical maintenance technicians to furnish continuous plant staffing. BENEFITS: R.J. Reynolds Tobacco Company now has on-site utility expertise motivated to continually reduce cost and focus on utility system reliability at two separate manufacturing locations. They also have access to all of Armstrong’s extensive utility/energy engineering resources including the following: • Annual engineering audits • Identification/development -

Tobacco Promotions
TOBACCO PROMOTIONS 10/1/2021 CHEWING TOBACCO ITEM # ITEM DESCRIPTION PACK SIZE 316500 BIG DUKE SOUTHERN BLEND $9.99 PP (16 OZ) 6 16 OZ 316502 BIG DUKE SOUTHERN BLEND $1.99PP SCRAP 12 3 OZ 316508 BIG DUKE SWEET BLD $1.99PP SCRAP 12 3 OZ 316513 BIG DUKE $1.99PP SCRAP 12 3 OZ 316515 STOKER'S RED SUPREME $1.99 PP 12 3 OZ 316521 BIG DUKE $9.99 PP (16 OZ) 6 16 OZ 316523 STOKER'S RED SUPREME BOLD $1.99 PP 12 3 OZ 320568 STARR PEACH VALUE SCRAP $2.19 PP 12 LARGE 320573 STARR PEACH SCRAP B1G1F 6 2 PKS 320574 STARR SCRAP $2.19 PP 12 LRG 320577 STARR SCRAP B1G1F 6 2 PKS 320964 BOWIE CHEW B1G1F 6 2 PKS 323265 LANCASTER B1G1F 6 2 PKS 323904 CHATTANOOGA CHEW $1.99 PP 12 3'S 323906 BOWIE CHEW $1.99 PP 12 LARGE MOIST SNUFF ITEM # ITEM DESCRIPTION PACK SIZE 321061 TIMBER WOLF PEACH PCH 1.79 ROLL 5 cans MOIST SNUFF (FLORIDA CUSTOMERS ONLY) ITEM # ITEM DESCRIPTION PACK SIZE 322043 TIMBER WOLF WG PCH 2.99 DISP 5 CANS 322045 TIMBER WOLF NAT PCH 2.99 DISP 5 CANS 322047 TIMBER WOLF MT PCH 2.99 DISP 5 CANS 322049 TIMBER WOLF PEACH PCH 2.99 DISP 5 CANS 322051 LONGHORN WG PCH 2.99 DISP 5 CANS 322053 LONGHORN ST PCH 2.99 DISP 5 CANS 322055 LONGHORN NAT PCH 2.99 DISP 5 CANS 322057 LONGHORN MT PCH 2.99 DISP 5 CANS 322082 LONGHORN LC WG 2.99 10 CANS 322084 LONGHORN FCN 2.99 10 CANS 322086 LONGHORN LC ST 2.99 10 CANS 322088 LONGHORN LC MT 2.99 10 CANS Page 1 of 5 NEW E-CIG ITEMS ITEM # ITEM DESCRIPTION PACK SIZE 399007 VUSE SOLO POWER UNIT 5 EACH 399083 VUSE CIRO POWER UNIT 5 EACH 399151 VUSE ALTO POWER UNIT BLUE 5 EACH 399153 VUSE ALTO POWER UNIT GOLD 5 EACH 399156 -

Voluntary Product Recall on Specified VUSE VIBE Products
Voluntary Product Recall – specific actions required Please review and execute all instructions below CC-80-18 April 13, 2018 To Our Retail and Subjobber Customers Selling VUSE VIBE Products SUBJECT: Voluntary Product Recall Issued on Specified VUSE VIBE Products • This notice is to alert retail and subjobber customers that R. J. Reynolds Vapor Company (RJRV) has voluntarily issued a nationwide safety recall on VUSE VIBE products containing the VIBE Power Unit (VIBE Kits and VIBE Power Units) after receiving consumer complaints about malfunctioning batteries causing the power unit to overheat. RJRV has notified the U. S. Food and Drug Administration and will be working with them on this voluntary recall. − A copy of the press release from RJRV regarding this matter is attached for your reference. Vuse Vibe Recall Press Release.pdf • The voluntary product recall applies to VUSE VIBE products containing the VIBE Power Unit (VIBE Kits and VIBE Power Unit Only). • No other RJRV products (e.g. VUSE VIBE Tanks, VUSE SOLO or VUSE Ciro Power Units or Cartridges) are included within this voluntary product recall. − VUSE SOLO and VUSE Ciro Power Units and Cartridges are NOT part of this voluntary product recall and should remain available for adult consumer purchase as normal. − VUSE VIBE Tanks are NOT affected by this voluntary product recall. VUSE VIBE Tank replenishment product will not be available from your supplier at this time. As part of this voluntary product recall, we are asking retail and subjobber customers to immediately take the following actions: 1. Immediately stop selling VUSE VIBE products (VIBE Kits, VIBE Power Units Only and VIBE Pre- Filled Tanks) to customers/consumers. -

Directory of Fire Safe Certified Cigarette Brand Styles Updated 4/29/10
Directory of Fire Safe Certified Cigarette Brand Styles Updated 4/29/10 Beginning August 1, 2008, only the cigarette brands and styles listed below are allowed to be imported, stamped and/or sold in the State of Alaska. Per AS 18.74, these brands must be marked as fire safe on the packaging. The brand styles listed below have been certified as fire safe by the State Fire Marshall, bear the "FSC" marking. There is an exception to these requirements. The new fire safe law allows for the sale of cigarettes that are not fire safe and do not have the "FSC" marking as long as they were stamped and in the State of Alaska before August 1, 2008 and found on the "Directory of MSA Compliant Cigarette & RYO Brands." Filter/ Non- Brand Style Length Circ. Filter Pkg. Descr. Manufacturer 1839 Full Flavor 82.7 24.60 Filter Hard Pack U.S. Flue-Cured Tobacco Growers, Inc. 1839 Full Flavor 97 24.60 Filter Hard Pack U.S. Flue-Cured Tobacco Growers, Inc. 1839 Full Flavor 83 24.60 Non-Filter Soft Pack U.S. Flue-Cured Tobacco Growers, Inc. 1839 Light 83 24.40 Filter Hard Pack U.S. Flue-Cured Tobacco Growers, Inc. 1839 Light 97 24.50 Filter Hard Pack U.S. Flue-Cured Tobacco Growers, Inc. 1839 Menthol 97 24.50 Filter Hard Pack U.S. Flue-Cured Tobacco Growers, Inc. 1839 Menthol 83 24.60 Filter Hard Pack U.S. Flue-Cured Tobacco Growers, Inc. 1839 Menthol Light 83 24.50 Filter Hard Pack U.S. -

Page 1 of 15
Updated September14, 2021– 9:00 p.m. Date of Next Known Updates/Changes: *Please print this page for your own records* If there are any questions regarding pricing of brands or brands not listed, contact Heather Lynch at (317) 691-4826 or [email protected]. EMAIL is preferred. For a list of licensed wholesalers to purchase cigarettes and other tobacco products from - click here. For information on which brands can be legally sold in Indiana and those that are, or are about to be delisted - click here. *** PLEASE sign up for GovDelivery with your EMAIL and subscribe to “Tobacco Industry” (as well as any other topic you are interested in) Future lists will be pushed to you every time it is updated. *** https://public.govdelivery.com/accounts/INATC/subscriber/new RECENTLY Changed / Updated: 09/14/2021- Changes to LD Club and Tobaccoville 09/07/2021- Update to some ITG list prices and buydowns; Correction to Pall Mall buydown 09/02/2021- Change to Nasco SF pricing 08/30/2021- Changes to all Marlboro and some RJ pricing 08/18/2021- Change to Marlboro Temp. Buydown pricing 08/17/2021- PM List Price Increase and Temp buydown on all Marlboro 01/26/2021- PLEASE SUBSCRIBE TO GOVDELIVERY EMAIL LIST TO RECEIVE UPDATED PRICING SHEET 6/26/2020- ***RETAILER UNDER 21 TOBACCO***(EFF. JULY 1) (on last page after delisting) Minimum Minimum Date of Wholesale Wholesale Cigarette Retail Retail Brand List Manufacturer Website Price NOT Price Brand Price Per Price Per Update Delivered Delivered Carton Pack Premier Mfg. / U.S. 1839 Flare-Cured Tobacco 7/15/2021 $42.76 $4.28 $44.00 $44.21 Growers Premier Mfg. -

First-Of-Its-Kind Filing for VUSE Products
Reynolds American Inc. P.O. Box 2990 Winston-Salem, NC 27102-2990 Media Contact: Kaelan Hollon [email protected] 202-421-4921 VUSE Premarket Tobacco Product Application Filed for Substantive Review by the FDA First-of-its-Kind Filing for VUSE Products WINSTON-SALEM, N.C. – Nov. 27, 2019 – Today, Reynolds American Inc. (“RAI”) announced that the recently-submitted Premarket Tobacco Product Application (“PMTA”) for VUSE vapor products has been filed by the Food and Drug Administration (“FDA”) for substantive scientific review, moving the application one step further through the review process. “This is a first-of-its-kind application for VUSE products, and it puts VUSE one step closer to gaining a marketing order from the FDA. FDA will now review our scientific justification and determine the appropriateness of VUSE e-cigarette products against the public health standard,” notes RAI CEO Ricardo Oberlander. “For adult tobacco consumers seeking an alternative source of nicotine, having FDA oversight of e-cigarette products is an important step to ensure those alternatives meet strict regulatory scrutiny.” The FDA’s scientific review of the VUSE application – which totals more than 150,000 pages of research and data – comes just over six weeks after its initial submission to the FDA. The FDA, per its earlier-issued guidance, considers the following issues, among others: • Risks and benefits to the population as a whole, including both users and nonusers of tobacco products; • Whether people who currently use any tobacco product would be more or less likely to stop using such products if the proposed new tobacco product were available; • Whether people who currently do not use any tobacco products would be more or less likely to begin using tobacco products if the new product were available; and • The methods, facilities, and controls used to manufacture, process, and pack the new tobacco product.