International Exclusion Zones DPD EXPRESS
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annex a Italian “White List” Countries and Lists of Supranational Entities and Central Banks (Identified by Acupay System LLC As of July 1, 2014)
Important Notice The Depository Trust Company B #: 1361-14 Date: July 17, 2014 To: All Participants Category: Dividends From: International Services Attention: Operations, Reorg & Dividend Managers, Partners & Cashiers Tax Relief – Country: Italy Intesa Sanpaolo S.p.A. CUSIP: 46115HAP2 Subject: Record Date: 12/28/2014 Payable Date: 01/12/2015 EDS Cut-Off: 01/09/2015 8:00 P.M Participants can use DTC’s Elective Dividend System (EDS) function over the Participant Terminal System (PTS) or Tax Relief option on the Participant Browser System (PBS) web site to certify all or a portion of their position entitled to the applicable withholding tax rate. Participants are urged to consult the PTS or PBS function TAXI or TaxInfo respectively before certifying their elections over PTS or PBS. Important: Prior to certifying tax withholding elections, participants are urged to read, understand and comply with the information in the Legal Conditions category found on TAXI or TaxInfo in PTS or PBS respectively. ***Please read this Important Notice fully to ensure that the self-certification document is sent to the agent by the indicated deadline*** Questions regarding this Important Notice may be directed to Acupay 212-422-1222. Important Legal Information: The Depository Trust Company (“DTC”) does not represent or warrant the accuracy, adequacy, timeliness, completeness or fitness for any particular purpose of the information contained in this communication, which is based in part on information obtained from third parties and not independently verified by DTC and which is provided as is. The information contained in this communication is not intended to be a substitute for obtaining tax advice from an appropriate professional advisor. -

Annual Report 2006
ANNUAL REPORT ON PORT STATE CONTROL IN THE ASIA-PACIFIC REGION 2006 This work is copyright. It may be reproduced in whole or part subject to the inclusion of an acknowledgement of the source but not for commercial use or sale. Further information may be obtained from: The Tokyo MOU Secretariat Ascend Shimbashi 8F 6-19-19 Shimbashi Minato-ku, Tokyo Japan 105-0004 Tel: +81-3-3433-0621 Fax: +81-3-3433-0624 This Report is also available at Tokyo MOU web-site (http://www.tokyo-mou.org) on Internet. MEMORANDUM OF UNDERSTANDING ON PORT STATE CONTROL IN THE ASIA-PACIFIC REGION FOREWORD We are pleased to present the Annual Report on Port State Control in the Asia-Pacific Region 2006. Tokyo MOU makes continuous endeavours and takes concerted measures to enhance and improve port State control activities in the region. In order to tackle on important areas relating to safety of navigation, safe operation of ships, ship security and prevention of pollution from ships and to facilitate and promote compliance of newly introduced convention regulations, Tokyo MOU embarks on concentrated inspection campaigns (CICs) periodically. A series of CICs have been conducted during the past and several further ones have been put on schedule for the coming years. This annual report covers the port State control developments and activities of the Tokyo MOU in 2006. Furthermore, the report also presents port State control statistics and analysis which provides the results of inspections carried out by member Authorities during the year. Successful and effective operation of the Tokyo MOU demonstrates the dedicated commitment of the eighteen member Authorities to combat against substandard shipping. -

Tax Relief Country: Italy Security: Intesa Sanpaolo S.P.A
Important Notice The Depository Trust Company B #: 15497-21 Date: August 24, 2021 To: All Participants Category: Tax Relief, Distributions From: International Services Attention: Operations, Reorg & Dividend Managers, Partners & Cashiers Tax Relief Country: Italy Security: Intesa Sanpaolo S.p.A. CUSIPs: 46115HAU1 Subject: Record Date: 9/2/2021 Payable Date: 9/17/2021 CA Web Instruction Deadline: 9/16/2021 8:00 PM (E.T.) Participants can use DTC’s Corporate Actions Web (CA Web) service to certify all or a portion of their position entitled to the applicable withholding tax rate. Participants are urged to consult TaxInfo before certifying their instructions over CA Web. Important: Prior to certifying tax withholding instructions, participants are urged to read, understand and comply with the information in the Legal Conditions category found on TaxInfo over the CA Web. ***Please read this Important Notice fully to ensure that the self-certification document is sent to the agent by the indicated deadline*** Questions regarding this Important Notice may be directed to Acupay at +1 212-422-1222. Important Legal Information: The Depository Trust Company (“DTC”) does not represent or warrant the accuracy, adequacy, timeliness, completeness or fitness for any particular purpose of the information contained in this communication, which is based in part on information obtained from third parties and not independently verified by DTC and which is provided as is. The information contained in this communication is not intended to be a substitute for obtaining tax advice from an appropriate professional advisor. In providing this communication, DTC shall not be liable for (1) any loss resulting directly or indirectly from mistakes, errors, omissions, interruptions, delays or defects in such communication, unless caused directly by gross negligence or willful misconduct on the part of DTC, and (2) any special, consequential, exemplary, incidental or punitive damages. -

Republic of Vanuatu Rapid Etrade Readiness Assessment
UNITED NATIONS CONFERENCE ON TRADE AND DEVELOPMENT Republic of Vanuatu Rapid eTrade Readiness Assessment Printed at United Nations, Geneva – 1820315 (E) – July 2018 – 500 – UNCTAD/DTL/STICT/2018/5 UNITED NATIONS CONFERENCE ON TRADE AND DEVELOPMENT Republic of Vanuatu Rapid eTrade Readiness Assessment New York and Geneva, 2018 ii Vanuatu Rapid eTrade Readiness Assessment © 2018, United Nations This work is available open access by complying with the Creative Commons licence created for intergovernmental organizations, available at http ://creativecommons.org/licenses/by/3.0/igo/. The findings, interpretations and conclusions expressed herein are those of the authors and do not necessarily reflect the views of the United Nations, its officials or Member States. The designation employed and the presentation of material on any map in this work do not imply the expression of any opinion whatsoever on the part of the United Nations concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Photocopies and reproductions of excerpts are allowed with proper credits. This publication has been edited externally. United Nations publication issued by the United Nations Conference on Trade and Development. UNCTAD/DTL/STICT/2018/5 NOTE iii NOTE Within the UNCTAD Division on Technology and Logistics, the ICT Policy Section carries out policy-oriented analytical work on the development implications of information and communication technologies (ICTs) and e-commerce. It is responsible for the preparation of the Information Economy Report (IER) as well as thematic studies on ICT for Development. The ICT Policy Section promotes international dialogue on issues related to ICTs for development and contributes to building developing countries’ capacities to measure the information economy and to design and implement relevant policies and legal frameworks. -

14879-21Tax Relief
Important Notice The Depository Trust Company B #: 14879‐21 Date: May 19, 2021 To: All Participants Category: Tax Relief, Distributions From: International Services Attention: Operations, Reorg & Dividend Managers, Partners & Cashiers Tax Relief – Country: Italy UniCredit S.p.A. CUSIP(s): 904678AG4 Subject: Record Date: 05/28/2021 Payable Date: 06/04/2021 CA Web Instruction Deadline: 06/03/2021 8:00 PM ET Participants can use DTC’s Corporate Actions Web (CA Web) service to certify all or a portion of their position entitled to the applicable withholding tax rate. Participants are urged to consult TaxInfo before certifying their instructions over CA Web. Important: Prior to certifying tax withholding instructions, participants are urged to read, understand and comply with the information in the Legal Conditions category found on TaxInfo over the CA Web. ***Please read this Important Notice fully to ensure that the self‐certification document is sent to the agent by the indicated deadline*** Questions regarding this Important Notice may be directed to Acupay at +1 212‐422‐1222. Important Legal Information: The Depository Trust Company (“DTC”) does not represent or warrant the accuracy, adequacy, timeliness, completeness or fitness for any particular purpose of the information contained in this communication, which is based in part on information obtained from third parties and not independently verified by DTC and which is provided as is. The information contained in this communication is not intended to be a substitute for obtaining tax advice from an appropriate professional advisor. In providing this communication, DTC shall not be liable for (1) any loss resulting directly or indirectly from mistakes, errors, omissions, interruptions, delays or defects in such communication, unless caused directly by gross negligence or willful misconduct on the part of DTC, and (2) any special, consequential, exemplary, incidental or punitive damages. -
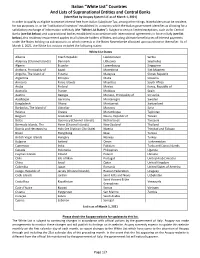
Countries and Lists of Supranational Entities and Central Banks
Italian “White List” Countries And Lists of Supranational Entities and Central Banks (Identified by Acupay System LLC as of March 1, 2021) In order to qualify as eligible to receive Interest free from Italian Substitute Tax, among other things, Noteholders must be resident, for tax purposes, in, or be "institutional investors" established in, a country which the Italian government identifies as allowing for a satisfactory exchange of information with Italy (the “White List States”). Subject to certain limited exceptions, such as for Central Banks (see list below) and supranational bodies established in accordance with international agreements in force in Italy (see list below), this residency requirement applies to all ultimate holders of Notes, including ultimate beneficiaries of Interest payments under the Notes holding via sub-accounts to which interests in the Notes Novemberbe allocated upon purchase or thereafter. As of March 1, 2021, the White List nations included the following states: White List States Albania Czech Republic Liechtenstein Serbia Alderney (Channel Islands) Denmark Lithuania Seychelles Algeria Ecuador Luxembourg Singapore Andorra, Principality of Egypt Macedonia Sint Maarten Anguilla, The Island of Estonia Malaysia Slovak Republic Argentina Ethiopia Malta Slovenia Armenia Faroe Islands Mauritius South Africa Aruba Finland Mexico Korea, Republic of Australia France Moldova Spain Austria Georgia Monaco, Principality of Sri Lanka Azerbaijan Germany Montenegro Sweden Bangladesh Ghana Montserrat Switzerland Barbados, -

2020 Inspection Results and Adopted New Performance Lists for Flag States and Recognized Organizations (Ros)
Press release 15 June 2021 Performance lists Paris MoU The Paris MoU Committee approved at its 54th me eting the 2020 inspection results and adopted new performance lists for flag States and Recognized Organizations (ROs). These lists will take effect from the 1st of July 2021. The “White, Grey and Black (WGB) List” presents the full spectrum, from quality flags to flags with a poor performance that are considered high or very high risk. It is based on the total number of inspections and detentions during a 3-year rolling period for flags with at least 30 inspections in the period. The “White List” represents quality flags with a consistently low detention record. Flags with an average performance are shown on the “Grey List”. Their appearance on this list may serve as an incentive to improve and move to the “White List”. At the same time flags at the lower end of the “Grey List” should be careful not to neglect control over their ships and risk ending up on the “Black List” next year. Regarding the “White, Grey and Black List” for 2020, a total number of 70 flags are listed: 39 on the “White List”, 22 on the “Grey List” and 9 on the “Black List”. In 2019 the total number of flag States on the list was also 70 of which 41 on the “White List”, 16 on the “Grey List” and 13 on the “Black List”. For several years the Committee has closely monitored the performance of ROs acting on behalf of flag States. To calculate the performance of ROs, the same formula to calculate the excess factor of the flags is used. -

Ship Registration and Related Matters
Guide to Ship Registration And Related Matters GUIDE TO SHIP REGISTRATION AND RELATED MATTERS Limassol, 2018 SHIPPING DEPUTY MINISTRY TO THE PRESIDENT REPUBLIC OF CYPRUS www.shipping.gov.cy Why Cyprus A maritime nation committed to a safe, secure and sustainable maritime industry 35˚Navigating 33˚Excellence • A sovereign flag of progress and quality. • The 3rd largest fleet in the EU. • Classified in the ‘White List’ of the Paris and Tokyo MoUs on Port State Control. • Business friendly common law jurisdiction. • Comprehensive and favourable Tonnage Tax System, approved by the European Union. • More than 60 Double Tax Avoidance Treaties. • More than 25 Bilateral Agreements on Merchant Shipping. • Competitive ship registration costs and fees. • No nationality restrictions for seafarers. • Top quality public services to the shipping industry 24/7. • Customer-oriented approach. • Value for money professional services of excellent quality. • Web services for the registration of seafarers and recognition of Certificates of Competency. • Maritime offices in Piraeus, London, Hamburg, Rotterdam, New York City and Brussels. • Strong involvement in international shipping fora (IMO, ILO) and EU. Member of the IMO Council since 1987. Index 9 | Preface 10 | Why the Cyprus flag? 12 | 1 Maritime Centre 13 | 2 Maritime Administration 14 | 3 Legislative framework (Overview) 16 | 4 Registration of ships 4.1 Types of registration 4.2 Overview 4.3 Conditions of ownership 4.4 Government Policy on the Registration of Ships under the Cyprus flag 4.5 Language -

Transferring Personal Data in Asia
Transferring Personal Data in Asia: A path to legal certainty and regional convergence This Comparative Review sets out proposals for how Asian public stakeholders may promote legal certainty and greater consistency between their respective laws and regulations on cross-border transfers of personal data in the region. Despite differences between the philosophies and the regulatory structures of each regime, there exist enough connecting points between national frameworks which lawmakers, governments, and data protection regulators can capitalize on, so as to promote and ensure responsible data flows between jurisdictions. Interoperability would be further enhanced by a common movement to align the standards by which legal grounds, mechanisms, and schemes for data transfers should be assessed. Alignment should be with a similarly high level of data protection so as to improve the situation of individuals and facilitate multi- jurisdictional compliance, as well as regulatory cooperation. Alignment not just to a regional standard but to global standards is a worthwhile goal, especially given the integration of Asian economies in global trade and the increased privacy expectations of the Asian public. This Review is supported by a comprehensive comparative Table of the rules relating to the transfer of personal data in 14 Asian jurisdictions. May 2020 Index Why this Review? ___________________________________________________________ 3 Key Findings _______________________________________________________________ 5 Collective Benefits of Legal -

Tokyo MOU Annual Report
ANNUAL REPORT ON PORT STATE CONTROL IN THE ASIA-PACIFIC REGION 2007 This work is copyright. It may be reproduced in whole or part subject to the inclusion of an acknowledgement of the source but not for commercial use or sale. Further information may be obtained from: The Tokyo MOU Secretariat Ascend Shimbashi 8F 6-19-19 Shimbashi Minato-ku, Tokyo Japan 105-0004 Tel: +81-3-3433-0621 Fax: +81-3-3433-0624 This Report is also available at Tokyo MOU web-site (http://www.tokyo-mou.org) on Internet. MEMORANDUM OF UNDERSTANDING ON PORT STATE CONTROL IN THE ASIA-PACIFIC REGION FOREWORD We are pleased to present the Annual Report on Port State Control in the Asia-Pacific Region 2007. Tokyo MOU continues to develop and enhance port State control activities in the region. Member Authorities of the Tokyo MOU once again rewrite the record of the previous year with the total number of inspections 22,039 in 2007. For verifying whether safety management system has been effectively established and properly maintained on board ships after five years of implementation, Tokyo MOU, together with the Paris MOU and other MOUs, conducted a concentrated inspection campaign (CIC) on the ISM Code in the fall of 2007. This annual report provides introduction of the port State control developments and activities of the Tokyo MOU in 2007. Moreover, the report also incorporates port State control statistics and analysis which summarizes the results of inspections carried out by member Authorities during the year. Continuous enforcement of port State control has received positive effect in improving quality of shipping in the region. -

Annual Report 2013
Port State Control Consolidating Progress THE PARIS MEMORANDUM OF UNDERSTANDING ON PORT STATE CONTROL STATE PORT ON UNDERSTANDING OF MEMORANDUM PARIS THE Annual Report 2013 Annual Report 2013 Contents Statement by Paris MoU chairman 4 Statement by the Secretary General 6 Executive summary 8 Paris MoU developments 10 Facts & Figures 2013 18 Statistical Annexes Annual Report 2013 23 White list 31 Grey List 33 Black List 35 Explanatory note - “White”, “Grey” and “Black List” 58 Secretariat Paris Memorandum of Understanding on Port State Control 60 ANNUAL REPORT 2013 3 PORT STATE CONTROL - CONSOLIDATING PROGRESS The year 2013 was a busy and important year for the Paris MoU as a Harmonized Verification Programme (HAVEP) was conducted on cruise liners and a Concentrated Inspection Campaign (CIC) on Propulsion and Auxiliary Machinery was carried out during inspections of other ship types. We will share the results of the HAVEP and CIC with the wider maritime transport community through the International Maritime Organization (IMO). Statement by the Paris MoU chairman This year also saw the entry into 2013: force of the International Labour Organization’s (ILO) Maritime a busy and important Labour Convention (MLC) in August. This was a key event in our industry year for the Paris MoU and the Convention will play an important part of port State control in the coming years. We held our Port State Control Committee’s 46th Meeting in Valetta, Malta, in May 2013. The meeting coincided with the European Maritime Day being held in Malta and this provided an opportunity to place the work of the Paris MoU in a wider context. -

Italy Enacts New “White List”
SEPTEMBER 2016 COMMENTARY Italy Enacts New “White List” On August 9, 2016 the Italian Ministry of Finance issued White List has added many new countries and territo- a Decree (“the Decree”)1 amending the list of countries ries, including some traditionally considered as “non- that are deemed to allow for an adequate exchange of cooperative” and, as such, historically “black listed” information with Italy, the so-called “White List” set out for Italian tax purposes. These include, for example, in Ministerial Decree of September 4, 1996. the Channel Islands (e.g., Jersey and Guernsey), cer- The Decree has substantially expanded the list of tain Caribbean islands (e.g., Anguilla, Aruba, Bermuda, white-listed countries: there are now 123 jurisdictions2 Curacao, Cayman Islands, British Virgin Islands) and in the White List, compared to approximately 70 pre- other European and non-European territories (e.g., viously. Pursuant to Decree No. 147 of September 14, the Isle of Man, Gibraltar, Liechtenstein, Switzerland, 2015, the White List is updated every six months and, Hong Kong, and Taiwan). With the entry into force of in this regard, the Decree states that jurisdictions that the Decree, the number of foreign investors who can should turn out not to allow, de facto, for an adequate in principle benefit from the Italian withholding and exchange of information with Italy will be subsequently other taxes exemptions provided for by the Italian tax removed from the White List (e.g., in case of continued statutes substantially increases. There are also some violations of the cooperation obligations provided for advantages for Italian tax resident persons that derive by the double tax treaties entered into with Italy).