ALKANE NAMES, Formulas, Properties (Memorize) (Sections 3.2,4)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemistry 0310 - Organic Chemistry 1 Chapter 3
Dr. Peter Wipf Chemistry 0310 - Organic Chemistry 1 Chapter 3. Reactions of Alkanes The heterolysis of covalent bonds yields anions and cations, whereas the homolysis creates radicals. Radicals are species with unpaired electrons that react mostly as electrophiles, seeking a single electron to complete their octet. Free radicals are important reaction intermediates and are formed in initiation reactions under conditions that cause the homolytic cleavage of bonds. In propagation steps, radicals abstract hydrogen or halogen atoms to create new radicals. Combinations of radicals are rare due to the low concentration of these reactive intermediates and result in termination of the radical chain. !CHAIN REACTION SUMMARY reactant product initiation PhCH3 HCl Cl 2 h DH = -16 kcal/mol chain-carrying intermediates n o r D (low concentrations) PhCH2 . Cl . propagation PhCH2 . or Cl . PhCH . 2 DH = -15 kcal/mol or Cl . PhCH2Cl or PhCH CH Ph PhCH2Cl Cl2 2 2 PhCH2Cl or Cl2 termination product reactant termination Alkanes are converted to alkyl halides by free radical halogenation reactions. The relative stability of radicals is increased by conjugation and hyperconjugation: R H H H . CH2 > R C . > R C . > H C . > H C . R R R H Oxygen is a diradical. In the presence of free-radical initiators such as metal salts, organic compounds and oxygen react to give hydroperoxides. These autoxidation reactions are responsible for the degradation reactions of oils, fatty acids, and other biological substances when exposed to air. Antioxidants such as hindered phenols are important food additives. Vitamins E and C are biological antioxidants. Radical chain reactions of chlorinated fluorocarbons in the stratosphere are responsible for the "ozone hole". -

Polyphenolic Compounds Extracted and Purified from Buddleja Globosa
molecules Article Polyphenolic Compounds Extracted and Purified from Buddleja Globosa Hope (Buddlejaceae) Leaves Using Natural Deep Eutectic Solvents and Centrifugal Partition Chromatography Jeniffer Torres-Vega 1 , Sergio Gómez-Alonso 2 , José Pérez-Navarro 2 , Julio Alarcón-Enos 3 and Edgar Pastene-Navarrete 1,3,* 1 Laboratorio de Farmacognosia, Departamento de Farmacia, Facultad de Farmacia, Universidad de Concepción, Concepción PC4030000, Chile; [email protected] 2 Regional Institute for Applied Scientific Research, Faculty of Chemical Sciences, University of Castilla-La Mancha, PC13071 Castilla-La Mancha, Spain; [email protected] (S.G.-A.); [email protected] (J.P.-N.) 3 Laboratorio de Síntesis y Biotransformación de Productos Naturales, Universidad del Bío-Bío, Chillán PC3800708, Chile; [email protected] * Correspondence: [email protected]; Tel.: +56-(42)-246-3000 Abstract: Chemical profiling of Buddleja globosa was performed by high-performance liquid chro- matography coupled to electrospray ionization (HPLC-DAD-ESI-IT/MS) and quadrupole time-of-flight high-resolution mass spectrometry (HPLC-ESI-QTOF/MS). The identification of 17 main phenolic com- pounds in B. globosa leaf extracts was achieved. Along with caffeoyl glucoside isomers, caffeoylshikimic Citation: Torres-Vega, J.; acid and several verbascoside derivatives (β-hydroxyverbascoside and β-hydroxyisoverbascoside) were Gómez-Alonso, S.; Pérez-Navarro, J.; Alarcón-Enos, J.; Pastene-Navarrete, identified. Among flavonoid compounds, the presence of 6-hydroxyluteolin-7-O-glucoside, quercetin-3- E. Polyphenolic Compounds O-glucoside, luteolin 7-O-glucoside, apigenin 7-O-glucoside was confirmed. Campneoside I, forsytho- Extracted and Purified from Buddleja side B, lipedoside A and forsythoside A were identified along with verbascoside, isoverbascoside, Globosa Hope (Buddlejaceae) Leaves eukovoside and martynoside. -

Electron Ionization
Chapter 6 Chapter 6 Electron Ionization I. Introduction ......................................................................................................317 II. Ionization Process............................................................................................317 III. Strategy for Data Interpretation......................................................................321 1. Assumptions 2. The Ionization Process IV. Types of Fragmentation Pathways.................................................................328 1. Sigma-Bond Cleavage 2. Homolytic or Radical-Site-Driven Cleavage 3. Heterolytic or Charge-Site-Driven Cleavage 4. Rearrangements A. Hydrogen-Shift Rearrangements B. Hydride-Shift Rearrangements V. Representative Fragmentations (Spectra) of Classes of Compounds.......... 344 1. Hydrocarbons A. Saturated Hydrocarbons 1) Straight-Chain Hydrocarbons 2) Branched Hydrocarbons 3) Cyclic Hydrocarbons B. Unsaturated C. Aromatic 2. Alkyl Halides 3. Oxygen-Containing Compounds A. Aliphatic Alcohols B. Aliphatic Ethers C. Aromatic Alcohols D. Cyclic Ethers E. Ketones and Aldehydes F. Aliphatic Acids and Esters G. Aromatic Acids and Esters 4. Nitrogen-Containing Compounds A. Aliphatic Amines B. Aromatic Compounds Containing Atoms of Nitrogen C. Heterocyclic Nitrogen-Containing Compounds D. Nitro Compounds E. Concluding Remarks on the Mass Spectra of Nitrogen-Containing Compounds 5. Multiple Heteroatoms or Heteroatoms and a Double Bond 6. Trimethylsilyl Derivative 7. Determining the Location of Double Bonds VI. Library -
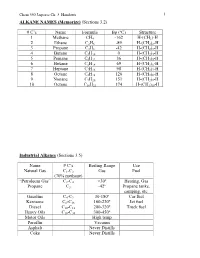
C's Name Formula Bp (ºC) Structure 1 Methane CH4 -162 H-(CH2)
Chem 350 Jasperse Ch. 3 Handouts 1 ALKANE NAMES (Memorize) (Sections 3.2) # C’s Name Formula Bp (ºC) Structure 1 Methane CH4 -162 H-(CH2)-H 2 Ethane C2H6 -89 H-(CH2)2-H 3 Propane C3H8 -42 H-(CH2)3-H 4 Butane C4H10 0 H-(CH2)4-H 5 Pentane C5H12 36 H-(CH2)5-H 6 Hexane C6H14 69 H-(CH2)6-H 7 Heptane C7H16 98 H-(CH2)7-H 8 Octane C8H18 126 H-(CH2)8-H 9 Nonane C9H20 151 H-(CH2)9-H 10 Octane C10H22 174 H-(CH2)10-H Industrial Alkanes (Sections 3.5) Name # C’s Boiling Range Use Natural Gas C1-C3 Gas Fuel (70% methane) “Petroleum Gas” C2-C4 <30º Heating, Gas Propane C3 -42º Propane tanks, camping, etc. Gasoline C4-C9 30-180º Car fuel Kerosene C8-C16 160-230º Jet fuel Diesel C10-C18 200-320º Truck fuel Heavy Oils C16-C30 300-450º Motor Oils High temp Paraffin Vacuum Asphalt Never Distills Coke Never Distills Chem 350 Jasperse Ch. 3 Handouts 2 Nomenclature of Alkanes (Sections 3.3) Systematic IUPAC Rules for Branched and Substituted Alkanes (Section 3.3B) 1. Longest continuous C-chain “core name” 2. Number core chain from an end nearest a substituent 3. Name substituents as “alkyl” groups: 4. Specify the location of substituents using numbers (hyphenate the #’s) • If >2 substituents, list alphabetically • Use di-, tri-, tetra- if the same substituent is repeated. (But ignore these in alphabetizing). Punctuation Notes: • Hyphenate numbers • Do not put a space between substituents and the core name Special Names for Some 3 or 4-carbon Substituents H3C CH3 CH Memorize H3C C H3C CH3 Isopropyl t-butyl or tert-butyl H2 H2 CH3 H3C C H3C C CH H3C CH Others C C C C CH3 H3C C H2 H H H2 2 2 H2 n-propyl n-butyl isobutyl s-butyl (n for "normal") Another Classification System Primary (1º): with one attached carbon Secondary (2º): with two attached carbons Tertiary (3º): with three attached carbons H C C C C C C C C H 1º H 2º C 3º Very Complex Substituents (Not responsible) Substituent: (1-ethyl-2,3-dimethylpentyl) Overall: 9-(1-ethyl-2,3-dimethylpentyl)nonadecane Chem 350 Jasperse Ch. -

Influence of Conjugation Axis on the Optical and Electronic Properties Of
Article pubs.acs.org/joc Influence of Conjugation Axis on the Optical and Electronic Properties of Aryl-Substituted Benzobisoxazoles † ‡ ‡ † † Brian C. Tlach, Aimeé L. Tomlinson, Alden G. Ryno, Dawn D. Knoble, Dana L. Drochner, † Kyle J. Krager, and Malika Jeffries-EL*, † Department of Chemistry, Iowa State University, Ames, Iowa 50010, United States ‡ Department of Chemistry, University of North Georgia, Dahlonega, Georgia 30597, United States *S Supporting Information ABSTRACT: Six different 2,6-diethyl-4,8-diarylbenzo[1,2-d:4,5-d′]bis(oxazoles) and four different 2,4,6,8-tetraarylbenzobisoxazoles were synthesized in two steps: a Lewis acid catalyzed orthoester cyclization followed by a Suzuki or Stille cross-coupling with various arenes. The influence of aryl group substitution and/or conjugation axis variation on the optical and electronic properties of these benzobis(oxazole) (BBO) compounds was evaluated. Structural modifications could be used to alter the HOMO, LUMO, and band gap over a range of 1.0, 0.5, and 0.5 eV, respectively. However, depending on the location and identity of the substituent, the HOMO level can be altered without significantly impacting the LUMO level. This is supported by the calculated frontier molecular orbitals. Our results indicate that the FMOs and band gaps of benzobisoxazoles can be readily modified either jointly or individually. ■ INTRODUCTION Among the aforementioned examples, the benzo[1,2-d:4,5- d′]bis(oxazole) (BBO)-based cruciforms are particularly During the past four decades, interest in the development of π- interesting, since these molecules have two different con- conjugated materials has increased due to their potential use as jugation pathways: 2,6-conjugation through the oxazole rings replacements for inorganic materials in a variety of semi- and 4,8-conjugation through the central benzene ring (Scheme conducting applications including field effect transistors − − 1). -

Introduction to Alkenes and Alkynes in an Alkane, All Covalent Bonds
Introduction to Alkenes and Alkynes In an alkane, all covalent bonds between carbon were σ (σ bonds are defined as bonds where the electron density is symmetric about the internuclear axis) In an alkene, however, only three σ bonds are formed from the alkene carbon -the carbon thus adopts an sp2 hybridization Ethene (common name ethylene) has a molecular formula of CH2CH2 Each carbon is sp2 hybridized with a σ bond to two hydrogens and the other carbon Hybridized orbital allows stronger bonds due to more overlap H H C C H H Structure of Ethylene In addition to the σ framework of ethylene, each carbon has an atomic p orbital not used in hybridization The two p orbitals (each with one electron) overlap to form a π bond (p bonds are not symmetric about the internuclear axis) π bonds are not as strong as σ bonds (in ethylene, the σ bond is ~90 Kcal/mol and the π bond is ~66 Kcal/mol) Thus while σ bonds are stable and very few reactions occur with C-C bonds, π bonds are much more reactive and many reactions occur with C=C π bonds Nomenclature of Alkenes August Wilhelm Hofmann’s attempt for systematic hydrocarbon nomenclature (1866) Attempted to use a systematic name by naming all possible structures with 4 carbons Quartane a alkane C4H10 Quartyl C4H9 Quartene e alkene C4H8 Quartenyl C4H7 Quartine i alkine → alkyne C4H6 Quartinyl C4H5 Quartone o C4H4 Quartonyl C4H3 Quartune u C4H2 Quartunyl C4H1 Wanted to use Quart from the Latin for 4 – this method was not embraced and BUT has remained Used English order of vowels, however, to name the groups -

C11 Aromatic and N-Alkane Hydrocarbons on Crete, in Air from Eastern Europe During the MINOS Campaign
Atmos. Chem. Phys., 3, 1461–1475, 2003 www.atmos-chem-phys.org/acp/3/1461/ Atmospheric Chemistry and Physics GC×GC measurements of C7−C11 aromatic and n-alkane hydrocarbons on Crete, in air from Eastern Europe during the MINOS campaign X. Xu1, J. Williams1, C. Plass-Dulmer¨ 2, H. Berresheim2, G. Salisbury1, L. Lange1, and J. Lelieveld1 1Max Planck Institute for Chemistry, Mainz, Germany 2German Weather Service, Meteorological Observatory Hohenpeissenberg, Germany Received: 23 January 2003 – Published in Atmos. Chem. Phys. Discuss.: 17 March 2003 Revised: 8 August 2003 – Accepted: 2 September 2003 – Published: 23 September 2003 Abstract. During the Mediterranean Intensive Oxidant campaign are estimated using the sequential reaction model Study (MINOS) campaign in August 2001 gas-phase or- and related data. They lie in the range of about 0.5–2.5 days. ganic compounds were measured using comprehensive two- dimensional gas chromatography (GC×GC) at the Finokalia ground station, Crete. In this paper, C7−C11 aromatic 1 Introduction and n-alkane measurements are presented and interpreted. The mean mixing ratios of the hydrocarbons varied from Atmospheric volatile organic compounds (VOCs) are recog- 1±1 pptv (i-propylbenzene) to 43±36 pptv (toluene). The nized as important atmospheric species affecting air chem- observed mixing ratios showed strong day-to-day variations istry on regional and global scales. Photochemical reactions and generally higher levels during the first half of the cam- of hydrocarbons in the atmosphere lead to the formation of paign. Mean diel profiles showed maxima at local mid- ozone, oxygenates and organic aerosols (Fehsenfeld et al., night and late morning, and minima in the early morning 1992; Andreae and Crutzen, 1997; Limbeck and Puxbaum, and evening. -

Reactions of Alkenes and Alkynes
05 Reactions of Alkenes and Alkynes Polyethylene is the most widely used plastic, making up items such as packing foam, plastic bottles, and plastic utensils (top: © Jon Larson/iStockphoto; middle: GNL Media/Digital Vision/Getty Images, Inc.; bottom: © Lakhesis/iStockphoto). Inset: A model of ethylene. KEY QUESTIONS 5.1 What Are the Characteristic Reactions of Alkenes? 5.8 How Can Alkynes Be Reduced to Alkenes and 5.2 What Is a Reaction Mechanism? Alkanes? 5.3 What Are the Mechanisms of Electrophilic Additions HOW TO to Alkenes? 5.1 How to Draw Mechanisms 5.4 What Are Carbocation Rearrangements? 5.5 What Is Hydroboration–Oxidation of an Alkene? CHEMICAL CONNECTIONS 5.6 How Can an Alkene Be Reduced to an Alkane? 5A Catalytic Cracking and the Importance of Alkenes 5.7 How Can an Acetylide Anion Be Used to Create a New Carbon–Carbon Bond? IN THIS CHAPTER, we begin our systematic study of organic reactions and their mecha- nisms. Reaction mechanisms are step-by-step descriptions of how reactions proceed and are one of the most important unifying concepts in organic chemistry. We use the reactions of alkenes as the vehicle to introduce this concept. 129 130 CHAPTER 5 Reactions of Alkenes and Alkynes 5.1 What Are the Characteristic Reactions of Alkenes? The most characteristic reaction of alkenes is addition to the carbon–carbon double bond in such a way that the pi bond is broken and, in its place, sigma bonds are formed to two new atoms or groups of atoms. Several examples of reactions at the carbon–carbon double bond are shown in Table 5.1, along with the descriptive name(s) associated with each. -

INVESTIGATION of POLYCYCLIC AROMATIC HYDROCARBONS (Pahs) on DRY FLUE GAS DESULFURIZATION (FGD) BY-PRODUCTS
INVESTIGATION OF POLYCYCLIC AROMATIC HYDROCARBONS (PAHs) ON DRY FLUE GAS DESULFURIZATION (FGD) BY-PRODUCTS DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Ping Sun, M.S. ***** The Ohio State University 2004 Dissertation Committee: Approved by Professor Linda Weavers, Adviser Professor Harold Walker Professor Patrick Hatcher Adviser Professor Yu-Ping Chin Civil Engineering Graduate Program ABSTRACT The primary goal of this research was to examine polycyclic aromatic hydrocarbons (PAHs) on dry FGD by-products to determine environmentally safe reuse options of this material. Due to the lack of information on the analytical procedures for measuring PAHs on FGD by-products, our initial work focused on analytical method development. Comparison of the traditional Soxhlet extraction, automatic Soxhlet extraction, and ultrasonic extraction was conducted to optimize the extraction of PAHs from lime spray dryer (LSD) ash (a common dry FGD by-product). Due to the short extraction time, ultrasonic extraction was further optimized by testing different organic solvents. Ultrasonic extraction with toluene as the solvent turned out to be a fast and efficient method to extract PAHs from LSD ash. The possible reactions of PAHs under standard ultrasonic extraction conditions were then studied to address concern over the possible degradation of PAHs by ultrasound. By sonicating model PAHs including naphthalene, phenanthrene and pyrene in organic solutions, extraction parameters including solvent type, solute concentration, and sonication time on reactions of PAHs were examined. A hexane: acetone (1:1 V/V) ii mixture resulted in less PAH degradation than a dichloromethane (DCM): acetone (1:1 V/V) mixture. -

Physical Organic Chemistry
PHYSICAL ORGANIC CHEMISTRY Yu-Tai Tao (陶雨台) Tel: (02)27898580 E-mail: [email protected] Website:http://www.sinica.edu.tw/~ytt Textbook: “Perspective on Structure and Mechanism in Organic Chemistry” by F. A. Corroll, 1998, Brooks/Cole Publishing Company References: 1. “Modern Physical Organic Chemistry” by E. V. Anslyn and D. A. Dougherty, 2005, University Science Books. Grading: One midterm (45%) one final exam (45%) and 4 quizzes (10%) homeworks Chap.1 Review of Concepts in Organic Chemistry § Quantum number and atomic orbitals Atomic orbital wavefunctions are associated with four quantum numbers: principle q. n. (n=1,2,3), azimuthal q.n. (m= 0,1,2,3 or s,p,d,f,..magnetic q. n. (for p, -1, 0, 1; for d, -2, -1, 0, 1, 2. electron spin q. n. =1/2, -1/2. § Molecular dimensions Atomic radius ionic radius, ri:size of electron cloud around an ion. covalent radius, rc:half of the distance between two atoms of same element bond to each other. van der Waal radius, rvdw:the effective size of atomic cloud around a covalently bonded atoms. - Cl Cl2 CH3Cl Bond length measures the distance between nucleus (or the local centers of electron density). Bond angle measures the angle between lines connecting different nucleus. Molecular volume and surface area can be the sum of atomic volume (or group volume) and surface area. Principle of additivity (group increment) Physical basis of additivity law: the forces between atoms in the same molecule or different molecules are very “short range”. Theoretical determination of molecular size:depending on the boundary condition. -

N-Alkanes and Polynuclear Aromatic Hydrocarbons (Pahs) in Water of Shatt Al-Arab River – Part 1 Makia M
G.J.B.A.H.S.,Vol.4(1):88-94 (January-March, 2015) ISSN: 2319 – 5584 Total Petroleum Hydrocarbons (TPHs) , n-alkanes and Polynuclear Aromatic Hydrocarbons (PAHs) in water of Shatt Al-Arab River – part 1 Makia M. Al-Hejuje * ; N. A. Hussain* ; & H. T. Al-saad ** *Department of Ecology , College of Sciences ,University of Basrah, Iraq . **Department of Marine Chemistry , Marine Science Center , University of Basrah, Iraq . Abstract Water samples were collected monthly from five stations along the middle part of Shatt Al Arab river during the low tide period from December , 2012 to November , 2013 to determine the concentrations , distribution and sources of hydrocarbons compounds in water samples. TPH is range from 5.18 µg/l to 37.59 µg/l . The carbon chain length of aliphatic ( n-alkanes) in water were recorded from C7 to C31 dominated by C22-C25 , and the total n-alkanes was ranged from 8.81 µg/l to 35.58 µg/l . The range of PAHs compounds was ( 5.81 – 47.96) ng/l ,dominated by carbazol and anthracene ( as light PAHs) and chrysene and floranthene (as heavy PAHs) . The LMW/HMW , CPI and Pristine/Phytane ratios indicated that the source of n-alkanes hydrocarbons was mainly biogenic and pyrogenic and at least petrogenic .Whereas the LMW/HMW , Phenanthrene /Anthracene , and Flouranthene / Pyrene ratios indicated that the source of PAHs compounds was mainly pyrogenic and petrogenic . Key words : Shatt Al-Arab water, TPHs , n-alkanes , PAHs , hydrocarbon indices. Introduction Tigris and the Euphrates rivers meet at Qurna town north of Basrah city and form the Shatt Al-Arab river with length of about 195 km , and width varies at different point from 0.35 km at Basrah city to 1.5 km at its mouth in Fao town . -

Physical Sciences Content
Foreword In order to improve learning outcomes the Department of Basic Education conducted research to determine the specific areas that learners struggle with in Grade 12 examinations. The research included a trend analysis by subject experts of learner performance over a period of five years as well as learner examination scripts in order to diagnose deficiencies or misconceptions in particular content areas. In addition, expert teachers were interviewed to determine the best practicesto ensure mastery of thetopic by learners and improve outcomes in terms of quality and quantity. The results of the research formed the foundation and guiding principles for the development of the booklets. In each identified subject, key content areas were identified for the development of material that will significantly improve learner's conceptual understanding whilst leading to improved performance in the subject. The booklets are developed as part of a series of booklets, with each bookletfocussing onlyon one specific challenging topic. The selected content is explained in detail and include relevant concepts from Grades 10 - 12 to ensure conceptual understanding. The main purpose of these booklets is to assist learners to master the content starting from a basic conceptual level of understanding to the more advanced level. The content in each booklets is presented in an easy to understand manner including the use of mind maps, summaries and exercises to support understanding and conceptual progression. These booklets should ideally be used as part of a focussed revision or enrichment program by learners after the topics have been taught in class. The booklets encourage learners to take ownership of their own learning and focus on developing and mastery critical content and skills such as reading and higher order thinking skills.