Chiodi Et Al 2019.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Chronology of Middle Missouri Plains Village Sites
Smithsonian Institution Scholarly Press smithsonian contributions to botany • n u m b e r 9 2 Smithsonian Institution Scholarly Press TaxonomicA Chronology Revision of of the MiddleChiliotrichum Missouri Group Plains Villagesensu stricto Sites (Compositae: Astereae) By Craig M. Johnson Joséwith Mauricio contributions Bonifacino by Stanley A. Ahler, Herbert Haas, and Georges Bonani SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of “diffusing knowledge” was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: “It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge.” This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, com- mencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Botany Smithsonian Contributions in History and Technology Smithsonian Contributions to the Marine Sciences Smithsonian Contributions to Museum Conservation Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology In these series, the Institution publishes small papers and full-scale monographs that report on the research and collections of its various museums and bureaus. The Smithsonian Contributions Series are distributed via mailing lists to libraries, universities, and similar institu- tions throughout the world. Manuscripts submitted for series publication are received by the Smithsonian Institution Scholarly Press from authors with direct affilia- tion with the various Smithsonian museums or bureaus and are subject to peer review and review for compliance with manuscript preparation guidelines. -

Dynamics of Large Pyroclastic Currents Inferred by the Internal Architecture of the Campanian Ignimbrite Claudio Scarpati*, Domenico Sparice & Annamaria Perrotta
www.nature.com/scientificreports OPEN Dynamics of large pyroclastic currents inferred by the internal architecture of the Campanian Ignimbrite Claudio Scarpati*, Domenico Sparice & Annamaria Perrotta Large ignimbrites are the product of devastating explosive eruptions that have repeatedly impacted climate and life on global scale. The assemblage of vertical and lateral lithofacies variations within an ignimbrite sheet, its internal architecture, may help to determine how the parental pyroclastic current evolves in time and space. The 39 ka Campanian Ignimbrite eruption, vented from Campi Flegrei caldera, laid down a thick ignimbrite over an area of thousands of km2. A detailed reconstruction of the vertical and lateral variation of the seven lithofacies recognised in the ignimbrite medial sequence constrains the behaviour of this event. The pyroclastic current fowed over a wide area around Campi Flegrei without depositing (bypass zone), and inundated a huge area during most of the paroxysmal, waxing phase, emplacing a mainly incipiently- to strongly- welded ignimbrite. Following this waxing phase, the leading edge of the current retreated back towards the source as the current waned, impacting a progressively smaller area and leaving an unconsolidated ash and lapilli deposit, later lithifed. Our study illustrates how large pyroclastic currents can evolve in time and space and the importance of both internal (eruptive and transport mechanisms) and external (topography, surfcial water and rain) factors in governing their behaviour. Catastrophic pyroclastic currents impact huge regions and represent one of the most devastating natural phenomena1. Large pyroclastic currents emplace thick sequences of ash- and vesiculated juvenile-rich deposits (ignimbrite2,3). Large ignimbrites show changes in facies on a regional scale 4. -

Appendix A. Supplementary Material to the Manuscript
Appendix A. Supplementary material to the manuscript: The role of crustal and eruptive processes versus source variations in controlling the oxidation state of iron in Central Andean magmas 1. Continental crust beneath the CVZ Country Rock The basement beneath the sampled portion of the CVZ belongs to the Paleozoic Arequipa- Antofalla terrain – a high temperature metamorphic terrain with abundant granitoid intrusions that formed in response to Paleozoic subduction (Lucassen et al., 2000; Ramos et al., 1986). In Northern Chile and Northwestern Argentina this Paleozoic metamorphic-magmatic basement is largely homogeneous and felsic in composition, consistent with the thick, weak, and felsic properties of the crust beneath the CVZ (Beck et al., 1996; Fig. A.1). Neodymium model ages of exposed Paleozoic metamorphic-magmatic basement and sediments suggest a uniform Proterozoic protolith, itself derived from intrusions and sedimentary rock (Lucassen et al., 2001). AFC Model Parameters Pervasive assimilation of continental crust in the Central Andean ignimbrite magmas is well established (Hildreth and Moorbath, 1988; Klerkx et al., 1977; Fig. A.1) and has been verified by detailed analysis of radiogenic isotopes (e.g. 87Sr/86Sr and 143Nd/144Nd) on specific systems within the CVZ (Kay et al., 2011; Lindsay et al., 2001; Schmitt et al., 2001; Soler et al., 2007). Isotopic results indicate that the CVZ magmas are the result of mixing between a crustal endmember, mainly gneisses and plutonics that have a characteristic crustal signature of high 87Sr/86Sr and low 145Nd/144Nd, and the asthenospheric mantle (low 87Sr/86Sr and high 145Nd/144Nd; Fig. 2). In Figure 2, we model the amount of crustal assimilation required to produce the CVZ magmas that are targeted in this study. -

Full-Text PDF (Final Published Version)
Pritchard, M. E., de Silva, S. L., Michelfelder, G., Zandt, G., McNutt, S. R., Gottsmann, J., West, M. E., Blundy, J., Christensen, D. H., Finnegan, N. J., Minaya, E., Sparks, R. S. J., Sunagua, M., Unsworth, M. J., Alvizuri, C., Comeau, M. J., del Potro, R., Díaz, D., Diez, M., ... Ward, K. M. (2018). Synthesis: PLUTONS: Investigating the relationship between pluton growth and volcanism in the Central Andes. Geosphere, 14(3), 954-982. https://doi.org/10.1130/GES01578.1 Publisher's PDF, also known as Version of record License (if available): CC BY-NC Link to published version (if available): 10.1130/GES01578.1 Link to publication record in Explore Bristol Research PDF-document This is the final published version of the article (version of record). It first appeared online via Geo Science World at https://doi.org/10.1130/GES01578.1 . Please refer to any applicable terms of use of the publisher. University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/red/research-policy/pure/user-guides/ebr-terms/ Research Paper THEMED ISSUE: PLUTONS: Investigating the Relationship between Pluton Growth and Volcanism in the Central Andes GEOSPHERE Synthesis: PLUTONS: Investigating the relationship between pluton growth and volcanism in the Central Andes GEOSPHERE; v. 14, no. 3 M.E. Pritchard1,2, S.L. de Silva3, G. Michelfelder4, G. Zandt5, S.R. McNutt6, J. Gottsmann2, M.E. West7, J. Blundy2, D.H. -
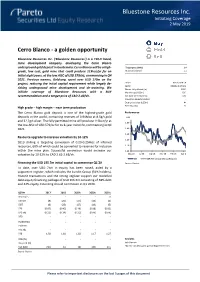
Bluestone Resources Inc. Initiating Coverage 2 May 2019
Bluestone Resources Inc. Initiating Coverage 2 May 2019 Cerro Blanco - a golden opportunity Bluestone Resources Inc. (‘Bluestone Resources’) is a TSX-V listed, mine development company, developing the Cerro Blanco underground gold deposit in Guatemala. Cerro Blanco will be a high- Target price (CAD) 2.4 grade, low cost, gold mine that could produce 113koz/yr for an Share price (CAD) 1.1 initial eight years, at the low AISC of USD 579/oz, commencing in Q4 2021. Previous owners, Goldcorp, spent over USD 170m on the project, reducing the initial capital requirement while largely de- Ticker BSR.V, BSR.CN Sector Metals & Mining risking underground mine development and de-watering. We Shares fully diluted (m) 103.7 initiate coverage of Bluestone Resources with a BUY Market cap (USDm) 63.5 recommendation and a target price of CAD 2.40/sh. Net debt Q1'19 (USDm) (20) Minority interests (USDm) - Enterprise value (USDm) 44 Free float (%) 33 High grade – high margin – near term production The Cerro Blanco gold deposit is one of the highest-grade gold Performance deposits in the world, containing reserves of 0.94Moz at 8.5g/t gold CAD and 32.2g/t silver. The fully permitted mine will produce 113koz/yr at 1.50 the low AISC of USD 579/oz for its 8-year mine life, commencing in Q4 2021. 1.40 1.30 Resource upgrade to increase valuation by 10-12% 1.20 2019 drilling is targeting conversion of 0.20-0.25Moz of inferred resources, 60% of which could be converted to reserves for inclusion 1.10 within the mine plan. -

Bianchi, M., Heit, B., Jakovlev, A., Yuan, X., Kay, SM
Originally published as: Bianchi, M., Heit, B., Jakovlev, A., Yuan, X., Kay, S.M., Sandvol, E., Alonso, R.N., Coira, B., Brown, L., Kind, R., Comte, D. (2013): Teleseismic tomography of the southern Puna plateau in Argentina and adjacent regions. ‐ Tectonophysics, 586, 65‐83 DOI: 10.1016/j.tecto.2012.11.016 *Manuscript Click here to download Manuscript: Bianchi-etal.doc Click here to view linked References Teleseismic tomography of the southern Puna plateau in Argentina and adjacent regions 1 2 3 M. Bianchi 1, B. Heit 1,*, A. Jakovlev 2, X. Yuan 1, S. M. Kay 3, E. Sandvol 4, R. N. Alonso 5, B. 4 5 6 Coira 6, R. Kind 1,7 7 8 9 1 Deutsches GeoForschungsZentrum GFZ, Telegrafenberg, 14473 Potsdam, Germany 10 11 12 13 2 Institute of Geology, SB RAS, Novosibirsk, Russia 14 15 16 3 Cornell University, EAS, Snee Hall, Ithaca, NY, 14850. 17 18 19 4 Department of Geological Sciences, University of Missouri, Columbia MO 65211 20 21 22 23 5 Universidad Nacional de Salta, Buenos Aires 177, 4400-Salta, Argentina 24 25 26 6 CONICET, Instituto de Geología y Minería, Universidad Nacional de Jujuy, Avda. Bolivia 1661, 27 28 29 4600-San Salvador de Jujuy, Argentina 30 31 32 7 Freie Universität Berlin, Maltesserstr. 74-100, 12227 Berlin, Germany 33 34 35 36 * Corresponding author, [email protected]. 37 38 39 40 41 42 43 ABSTRACT 44 45 46 An array of 74 seismological stations was deployed in the Argentine Puna and adjacent regions for 47 48 a period of two years. -

The Role of Crustal and Eruptive Processes Versus Source Variations in Controlling the Oxidation State of Iron in Central Andean Magmas ∗ Stephanie B
Earth and Planetary Science Letters 440 (2016) 92–104 Contents lists available at ScienceDirect Earth and Planetary Science Letters www.elsevier.com/locate/epsl The role of crustal and eruptive processes versus source variations in controlling the oxidation state of iron in Central Andean magmas ∗ Stephanie B. Grocke a,b, , Elizabeth Cottrell a, Shanaka de Silva b, Katherine A. Kelley c a Smithsonian Institution, National Museum of Natural History, Washington, DC 20560, USA b College of Earth, Ocean and Atmospheric Sciences, Oregon State University, Corvallis, OR 97331, USA c Graduate School of Oceanography, University of Rhode Island, Narragansett, RI 20882, USA a r t i c l e i n f o a b s t r a c t Article history: The composition of the continental crust is closely tied to subduction zone magmatism. Elevated oxygen Received 8 July 2015 fugacity ( f O2) plays a central role in fostering crystallization of oxide minerals and thereby aids in Received in revised form 21 January 2016 generating the calc-alkaline trend of iron depletion that characterizes the continents. Along continental Accepted 24 January 2016 margins, arc magmas erupt through continental crust and often undergo extensive differentiation that Available online 22 February 2016 may modify magmatic f O2. The importance of the subducting slab and mantle wedge relative to Editor: T.A. Mather the effects of this differentiation on the f O2 recorded by continental arc magmas remains relatively Keywords: unconstrained. Here, we focus on the effect of differentiation on magmatic f O2 using a suite of 14 Central Andean volcanic zone samples from the Central Volcanic Zone (CVZ) of the Andes where the continental crust is atypically thick crustal assimilation (60–80 km). -

Argentine National Report 1999-2003
REPÚBLICA ARGENTINA INFORME NACIONAL PRESENTADO A LA ASOCIACIÓN INTERNACIONAL DE VOLCANOLOGÍA Y QUÍMICA DEL INTERIOR DE LA TIERRA –IAVCEI– XXIV. ASAMBLEA GENERAL DE LA UNIÓN GEODÉSICA Y GEOFÍSICA INTERNACIONAL – UGGI – Perugia, Italia, 2 – 13 julio de 2007 -------------------- --------------------- ARGENTINA REPUBLIC NATIONAL REPORT PRESENTED TO THE INTERNATONAL ASSOCIATION OF VOLCANOLOGY AND CHEMISTRY OF THE EARTH INTERIOR –IAVCEI– XXIV GENERAL ASSEMBLY OF THE INTERNATIONAL UNION OF GEODESY AND GEOPHYSICS – IUGG – Perugia, Italy, 2 – 13 July, 2007 --------------------------------------------------------------------------------------------- COMITÉ NACIONAL DE LA UNIÓN GEODÉSICA Y GEOFÍSICA INTERNACIONAL NATIONAL COMMITTEE OF THE INTERNATIONAL UNION OF GEODESY AND GEOPHYSICS ------------------------------------------------------------------- ARGENTINA 2007 REPÚBLICA ARGENTINA COMITÉ NACIONAL DE LA UNIÓN GEODÉSICA Y GEOFÍSICA INTERNACIONAL (CNUGGI) ARGENTINA REPUBLIC NATIONAL COMMITTEE OF THE INTERNATIONAL UNION OF GEODESY AND GEOPHYSICS The main function of the IUGG National Committee is representing the Union in Argentina. It is chaired by the Military Geographic Institute Director, and assisted by two Vice Presidents, a General Secretary and a Secretary Assistant. The current (2007) head staff is as follows: President: Cnl. VGM Eng. Alfredo Augusto Stahlschmidt Executive 1st Vicepresident: Dr Corina Risso 2nd Vicepresident: Dr Nora Sabbione General Secretary: Surv. Rubén Carlos Ramos Secretary Assistant: Surv. Sergio Rubén Cimbaro Treasurer: -

Silicic Magma Chambers and Mafic Dikes a Dissertation Submitted to the Department Of
INVESTIGATIONS OF MAGMATIC END-MEMBERS: SILICIC MAGMA CHAMBERS AND MAFIC DIKES A DISSERTATION SUBMITTED TO THE DEPARTMENT OF GEOLOGICAL AND ENVIRONMENTAL SCIENCES AND THE COMMITTEE ON GRADUATE STUDIES OF STANFORD UNIVERSITY IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Gwyneth Retta Hughes May 2010 © 2010 by Gwyneth Retta Hughes. All Rights Reserved. Re-distributed by Stanford University under license with the author. This work is licensed under a Creative Commons Attribution- Noncommercial 3.0 United States License. http://creativecommons.org/licenses/by-nc/3.0/us/ This dissertation is online at: http://purl.stanford.edu/cf090yt6229 Includes supplemental files: 1. Caldera references for Chapters 2 and 3 (Caldera_index_ref.pdf) 2. Bayes Classifier Code for Chapter 3 (bayes_classifier.zip) 3. Caldera data for Chapter 2 (Arc_caldera_data.csv) 4. Caldera data for Chapter 3 (All_caldera_data.csv) ii I certify that I have read this dissertation and that, in my opinion, it is fully adequate in scope and quality as a dissertation for the degree of Doctor of Philosophy. Gail Mahood, Primary Adviser I certify that I have read this dissertation and that, in my opinion, it is fully adequate in scope and quality as a dissertation for the degree of Doctor of Philosophy. David Pollard I certify that I have read this dissertation and that, in my opinion, it is fully adequate in scope and quality as a dissertation for the degree of Doctor of Philosophy. Paul Segall Approved for the Stanford University Committee on Graduate Studies. Patricia J. Gumport, Vice Provost Graduate Education This signature page was generated electronically upon submission of this dissertation in electronic format. -

Lithospheric Delamination Beneath the Southern Puna Plateau Resolved by Local Earthquake Tomography
Confidential manuscript submitted to replace this text with name of AGU journal Lithospheric delamination beneath the southern Puna plateau resolved by local earthquake tomography Jing Chen1,2, Sofia-Katerina Kufner2,3, Xiaohui Yuan2, Benjamin Heit2, Hao Wu1, Dinghui Yang1, Bernd Schurr2, and Suzanne Kay4 1Tsinghua University, Beijing, China. 2Deutsches GeoForschungsZentrum GFZ, Potsdam, Germany. 3British Antarctica Survey, Cambridge, UK. 4Cornell University, Ithaca, USA Corresponding author: Jing Chen ([email protected]) Key Points: • A local earthquake tomography is performed in the southern Puna plateau and adjacent region • A high mantle Vp anomaly beneath Cerro Galan provides evidence for lithospheric delamination • A low Vp zone is observed beneath Ojos del Salado, reflecting the melt supplied by slab dehydration reaction at two depths This paper has been submitted for publication in Journal of Geophysical Research: Solid Earth. Confidential manuscript submitted to replace this text with name of AGU journal Abstract We present a local earthquake tomography to illuminate the crustal and uppermost mantle structure beneath the southern Puna plateau and to test the delamination hypothesis. Vp and Vp/Vs ratios were obtained using travel time variations recorded by 75 temporary seismic stations between 2007 and 2009. In the upper crust, prominent low Vp anomalies are found beneath the main volcanic centers, indicating the presence of magma and melt beneath the southern Puna plateau. In the lowlands to the southeast of the Puna plateau, below the Sierras Pampeanas, a high Vp body is observed in the crust. Beneath the Moho at around 90 km depth, a strong high Vp anomaly is detected just west of the giant backarc Cerro Galan Ignimbrite caldera with the robustness of this feature being confirmed by multiple synthetic tests. -
Volcanism in Reverse and Strike-Slip Fault Settings
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/227024325 Volcanism in Reverse and Strike-Slip Fault Settings Chapter · March 2010 DOI: 10.1007/978-90-481-2737-5_9 CITATIONS READS 17 450 3 authors, including: Alessandro Tibaldi Federico Pasquaré Mariotto Università degli Studi di Milano-Bicocca Università degli Studi dell'Insubria 151 PUBLICATIONS 2,600 CITATIONS 57 PUBLICATIONS 349 CITATIONS SEE PROFILE SEE PROFILE All content following this page was uploaded by Federico Pasquaré Mariotto on 04 February 2014. The user has requested enhancement of the downloaded file. All in-text references underlined in blue are added to the original document and are linked to publications on ResearchGate, letting you access and read them immediately. Volcanism in Reverse and Strike-Slip Fault Settings Alessandro Tibaldi, Federico Pasquarè, and Daniel Tormey Abstract Traditionally volcanism is thought to the volcano to the surface along the main faults, irre- require an extensional state of stress in the crust. This spective of the orientation of σ3. The petrology and review examines recent relevant data demonstrating geochemistry of lavas erupted in compressive stress that volcanism occurs also in compressional tectonic regimes indicate longer crustal residence times, and settings associated with reverse and strike-slip faulting. higher degrees of lower crustal and upper crustal melts Data describing the tectonic settings, structural analy- contributing to the evolving magmas when compared sis, analogue modelling, petrology, and geochemistry, to lavas from extensional stress regimes. Small vol- are integrated to provide a comprehensive presenta- umes of magma tend to rise to shallow crustal lev- tion of this topic. -

Magnesian Andesites and the Subduction Component in a Strongly Calc-Alkaline Series at Piip Volcano, Far Western Aleutians
0022-3530/94 $3.00 Magnesian Andesites and the Subduction Component in a Strongly Calc-Alkaline Series at Piip Volcano, Far Western Aleutians by G. M. YOGODZINSKI1, O. N. VOLYNETS2, A. V. KOLOSKOV2, 3 4 N. I. SELIVERSTOV , AND V. V. MATVENKOV Downloaded from 1 Department of Geological Sciences & INSTOC, Cornell University, Ithaca, New York 14850 2 The Institute of Volcanic Geology and Geochemistry, Petropavlovsk, Kamchatka 683006, Russia 3 The Institute of Volcanology, Petropavlovsk, Kamchatka, 683006, Russia http://petrology.oxfordjournals.org/ AThe Institute of Oceanology, Moscow, 117218, Russia (Received 6 July 1992; revised typescript accepted 6 April 1993) ABSTRACT Piip Volcano is a hydrothermally active seamount located in the strike-slip regime immediately north of the far Western Aleutian Ridge. Fractionation of hydrous and oxidized magnesian andesites (MA) produced an igneous rock series at Piip Volcano with a lower average FeO*/MgO (more strongly calc-alkaline) than any in the Central or Eastern Aleutian arc. Basaltic rocks in the Piip at Russian Archive 1 on December 8, 2016 Volcano area are rare, and those that do occur have characteristics transitional toward MA (high SiO2 and Na2O; low CaO/Al2O3). The compositions of the MA and their predominance as parental magmas throughout the Western Aleutians since Middle Miocene time suggest that transpressional tectonics causes primitive basaltic melts of the mantle wedge to pool immediately below the arc crust, where they interact with warm, ambient peridotite to produce highly silica-oversaturated lavas of mantle origin. Proposed consequences of a long melting column in the mantle wedge (e.g., high percentage melting and tholeiitic volcanism) are not observed at Piip Volcano, despite that fact that it is built on very thin crust.