Ecological Connectivity in Braided River-Scapes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Critical Habitat for Canterbury Freshwater Fish, Kōura/Kēkēwai and Kākahi
CRITICAL HABITAT FOR CANTERBURY FRESHWATER FISH, KŌURA/KĒKĒWAI AND KĀKAHI REPORT PREPARED FOR CANTERBURY REGIONAL COUNCIL BY RICHARD ALLIBONE WATERWAYS CONSULTING REPORT NUMBER: 55-2018 AND DUNCAN GRAY CANTERBURY REGIONAL COUNCIL DATE: DECEMBER 2018 EXECUTIVE SUMMARY Aquatic habitat in Canterbury supports a range of native freshwater fish and the mega macroinvertebrates kōura/kēkēwai (crayfish) and kākahi (mussel). Loss of habitat, barriers to fish passage, water quality and water quantity issues present management challenges when we seek to protect this freshwater fauna while providing for human use. Water plans in Canterbury are intended to set rules for the use of water, the quality of water in aquatic systems and activities that occur within and adjacent to aquatic areas. To inform the planning and resource consent processes, information on the distribution of species and their critical habitat requirements can be used to provide for their protection. This report assesses the conservation status and distributions of indigenous freshwater fish, kēkēwai and kākahi in the Canterbury region. The report identifies the geographic distribution of these species and provides information on the critical habitat requirements of these species and/or populations. Water Ways Consulting Ltd Critical habitats for Canterbury aquatic fauna Table of Contents 1 Introduction ......................................................................................................................................... 1 2 Methods .............................................................................................................................................. -
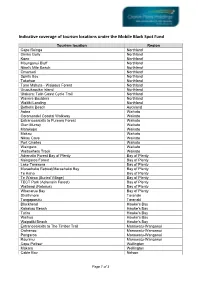
Indicative Coverage of Tourism Locations Under the Mobile Black Spot Fund
Indicative coverage of tourism locations under the Mobile Black Spot Fund Tourism location Region Cape Reinga Northland Glinks Gully Northland Kaeo Northland Maunganui Bluff Northland Ninety Mile Beach Northland Omamari Northland Spirits Bay Northland Takahue Northland Tane Mahuta - Waipoua Forest Northland Urupukapuka Island Northland Utakura: Twin Coast Cycle Trail Northland Wairere Boulders Northland Waitiki Landing Northland Bethells Beach Auckland Aotea Waikato Coromandel Coastal Walkway Waikato Entrances/exits to Pureora Forest Waikato Glen Murray Waikato Marokopa Waikato Mokau Waikato Nikau Cave Waikato Port Charles Waikato Waingaro Waikato Waitawheta Track Waikato Adrenalin Forest Bay of Plenty Bay of Plenty Kaingaroa Forest Bay of Plenty Lake Tarawera Bay of Plenty Maraehako Retreat/Maraehako Bay Bay of Plenty Te Kaha Bay of Plenty Te Wairoa (Buried Village) Bay of Plenty TECT Park (Adrenalin Forest) Bay of Plenty Waitangi (Rotorua) Bay of Plenty Whanarua Bay Bay of Plenty Strathmore Taranaki Tongaporutu Taranaki Blackhead Hawke's Bay Kairakau Beach Hawke's Bay Tutira Hawke's Bay Waihua Hawke's Bay Waipatiki Beach Hawke's Bay Entrances/exits to The Timber Trail Manawatu-Wanganui Owhango Manawatu-Wanganui Pongaroa Manawatu-Wanganui Raurimu Manawatu-Wanganui Cape Palliser Wellington Makara Wellington Cable Bay Nelson Page 1 of 3 Kenepuru Head Marlborough Okiwi Bay Marlborough Blue Lake/ Lake Rotoroa Tasman Cape Farewell Tasman Entrances/exits to Heaphy Track Tasman Lake Rotoroa Tasman Maruia Falls Tasman Totaranui Beach and campsite -

Geomorphic Classification of Rivers
9.36 Geomorphic Classification of Rivers JM Buffington, U.S. Forest Service, Boise, ID, USA DR Montgomery, University of Washington, Seattle, WA, USA Published by Elsevier Inc. 9.36.1 Introduction 730 9.36.2 Purpose of Classification 730 9.36.3 Types of Channel Classification 731 9.36.3.1 Stream Order 731 9.36.3.2 Process Domains 732 9.36.3.3 Channel Pattern 732 9.36.3.4 Channel–Floodplain Interactions 735 9.36.3.5 Bed Material and Mobility 737 9.36.3.6 Channel Units 739 9.36.3.7 Hierarchical Classifications 739 9.36.3.8 Statistical Classifications 745 9.36.4 Use and Compatibility of Channel Classifications 745 9.36.5 The Rise and Fall of Classifications: Why Are Some Channel Classifications More Used Than Others? 747 9.36.6 Future Needs and Directions 753 9.36.6.1 Standardization and Sample Size 753 9.36.6.2 Remote Sensing 754 9.36.7 Conclusion 755 Acknowledgements 756 References 756 Appendix 762 9.36.1 Introduction 9.36.2 Purpose of Classification Over the last several decades, environmental legislation and a A basic tenet in geomorphology is that ‘form implies process.’As growing awareness of historical human disturbance to rivers such, numerous geomorphic classifications have been de- worldwide (Schumm, 1977; Collins et al., 2003; Surian and veloped for landscapes (Davis, 1899), hillslopes (Varnes, 1958), Rinaldi, 2003; Nilsson et al., 2005; Chin, 2006; Walter and and rivers (Section 9.36.3). The form–process paradigm is a Merritts, 2008) have fostered unprecedented collaboration potentially powerful tool for conducting quantitative geo- among scientists, land managers, and stakeholders to better morphic investigations. -

Upper Riccarton Cemetery 2007 1
St Peter’s, Upper Riccarton, is the graveyard of owners and trainers of the great horses of the racing and trotting worlds. People buried here have been in charge of horses which have won the A. J. C. Derby, the V.R.C. Derby, the Oaks, Melbourne Cup, Cox Plate, Auckland Cup (both codes), New Zealand Cup (both codes) and Wellington Cup. Area 1 Row A Robert John Witty. Robert John Witty (‘Peter’ to his friends) was born in Nelson in 1913 and attended Christchurch Boys’ High School, College House and Canterbury College. Ordained priest in 1940, he was Vicar of New Brighton, St. Luke’s and Lyttelton. He reached the position of Archdeacon. Director of the British Sailors’ Society from 1945 till his death, he was, in 1976, awarded the Queen’s Service Medal for his work with seamen. Unofficial exorcist of the Anglican Diocese of Christchurch, Witty did not look for customers; rather they found him. He said of one Catholic lady: “Her priest put her on to me; they have a habit of doing that”. Problems included poltergeists, shuffling sounds, knockings, tapping, steps tramping up and down stairways and corridors, pictures turning to face the wall, cold patches of air and draughts. Witty heard the ringing of Victorian bells - which no longer existed - in the hallway of St. Luke’s vicarage. He thought that the bells were rung by the shade of the Rev. Arthur Lingard who came home to die at the vicarage then occupied by his parents, Eleanor and Archdeacon Edward Atherton Lingard. In fact, Arthur was moved to Miss Stronach’s private hospital where he died on 23 December 1899. -

Seasonal Flooding Affects Habitat and Landscape Dynamics of a Gravel
Seasonal flooding affects habitat and landscape dynamics of a gravel-bed river floodplain Katelyn P. Driscoll1,2,5 and F. Richard Hauer1,3,4,6 1Systems Ecology Graduate Program, University of Montana, Missoula, Montana 59812 USA 2Rocky Mountain Research Station, Albuquerque, New Mexico 87102 USA 3Flathead Lake Biological Station, University of Montana, Polson, Montana 59806 USA 4Montana Institute on Ecosystems, University of Montana, Missoula, Montana 59812 USA Abstract: Floodplains are comprised of aquatic and terrestrial habitats that are reshaped frequently by hydrologic processes that operate at multiple spatial and temporal scales. It is well established that hydrologic and geomorphic dynamics are the primary drivers of habitat change in river floodplains over extended time periods. However, the effect of fluctuating discharge on floodplain habitat structure during seasonal flooding is less well understood. We collected ultra-high resolution digital multispectral imagery of a gravel-bed river floodplain in western Montana on 6 dates during a typical seasonal flood pulse and used it to quantify changes in habitat abundance and diversity as- sociated with annual flooding. We observed significant changes in areal abundance of many habitat types, such as riffles, runs, shallow shorelines, and overbank flow. However, the relative abundance of some habitats, such as back- waters, springbrooks, pools, and ponds, changed very little. We also examined habitat transition patterns through- out the flood pulse. Few habitat transitions occurred in the main channel, which was dominated by riffle and run habitat. In contrast, in the near-channel, scoured habitats of the floodplain were dominated by cobble bars at low flows but transitioned to isolated flood channels at moderate discharge. -

Mount Cook Rally Action
NEW ZEALAND’S FOREMOST HISTORIC MOTORING MAGAZINE No. 259 DECEMBER 2002 /JANUARY 2003 PRICE $4.50 INCL GST Beaded Wheels 9 418979 000012 Mount Cook Rally Action bw259.indd 1 10/10/2007 11:05:37 PM This card-mounted photograph of an early Buick is stamped with the photographer’s signature which appears to be “Ballows, Wanganui”. VINTAGE & CLASSIC The car has FBC Taxi No is painted at the top of the driver’s door. The number which appears to end in a zero is partially obscured by the brake ENGINE PARTS lever. The headlamps appear to have seen better days and no nights, however the white topped forage cap worn by the driver indicates the car is still in service as a taxi. No number plate and no other information, can readers assist? SubmissionsPhoto of photographs supplied for bythis Barrypage are welcomeThomson. from Beaded Wheels Beaded Wheels readers. Please send original photographs of NEW ZEALAND’S FOREMOST HISTORIC MOTORING MAGAZINE historic interest with any available information to Beaded Wheels, PO Box 13140, Christchurch. PISTONS, VALVES, HEAD GASKETS Laserprints/photocopies are not suitable. Photos will be returned as soon as practicable. TIMING GEARS, MORSE CHAINS ENGINE BEARINGS GEORGE CALDER LIMITED 307 HOON HAY ROAD, CHRISTCHURCH PH 03 338 5372 FAX 03 338 5482 Stockists of REPLACEMENT 1912-80 AUTOMOTIVE ENGLISH PARTS AMERICAN CONTINENTAL Kingpin sets Engine gaskets Gearbox gears Suspension parts. Steering joints Crownwheel & pinions Example: 475-19 Spark plugs Electrical fittings Wiper motors (vac) 4ply Engine bearings Shock -

Natural Character, Riverscape & Visual Amenity Assessments
Natural Character, Riverscape & Visual Amenity Assessments Clutha/Mata-Au Water Quantity Plan Change – Stage 1 Prepared for Otago Regional Council 15 October 2018 Document Quality Assurance Bibliographic reference for citation: Boffa Miskell Limited 2018. Natural Character, Riverscape & Visual Amenity Assessments: Clutha/Mata-Au Water Quantity Plan Change- Stage 1. Report prepared by Boffa Miskell Limited for Otago Regional Council. Prepared by: Bron Faulkner Senior Principal/ Landscape Architect Boffa Miskell Limited Sue McManaway Landscape Architect Landwriters Reviewed by: Yvonne Pfluger Senior Principal / Landscape Planner Boffa Miskell Limited Status: Final Revision / version: B Issue date: 15 October 2018 Use and Reliance This report has been prepared by Boffa Miskell Limited on the specific instructions of our Client. It is solely for our Client’s use for the purpose for which it is intended in accordance with the agreed scope of work. Boffa Miskell does not accept any liability or responsibility in relation to the use of this report contrary to the above, or to any person other than the Client. Any use or reliance by a third party is at that party's own risk. Where information has been supplied by the Client or obtained from other external sources, it has been assumed that it is accurate, without independent verification, unless otherwise indicated. No liability or responsibility is accepted by Boffa Miskell Limited for any errors or omissions to the extent that they arise from inaccurate information provided by the Client or -

Wetlands Resource Has Been Developed to Encourage You and Your Class to Visit Wetlands in Your ‘Backyard’
WETLANDS for Education in the West Coast Tai Poutini Conservancy January 2005 Edition 2 WETLANDS for Education in the West Coast Tai Poutini Conservancy ACKNOWLEDGEMENTS A large number of people have been involved in the production and editing of this resource. We would like to thank them all and in particular the following: Area and Conservancy staff, especially Philippe Gerbeaux for their comments and assistance. Te Rüanga o Ngäi Tahu, Te Rünaka o Makäwhio and Te Rünaka o Ngäti Waewae for their comments and assistance through Rob Tipa. Compiled by Chrisie Sargent, Sharleen Hole and Kate Leggett Edited by Sharleen Hole and Julie Dutoit Illustrations by Sue Asplin ISBN 0-478-22656-X 2nd Edition January 2005 Published by: Department of Conservation Greymouth Mawheranui Area Office PO Box 370 Greymouth April 2004 CONTENTS USING THIS RESOURCE 5 THE CURRICULUM 5 WHY WETLANDS? 8 RAMSAR CONVENTION 9 SAFETY 9 BE PREPARED ACTIVITY 10 PRE VISIT ACTIVITIES 11 POST VISIT ACTIVITIES 12 FACT SHEETS What is a wetland? 15 Types of wetlands 17 How wetlands change over time 19 Threats to wetlands 21 Wetland uses & users 23 The water cycle is important for wetlands 24 Catchments 25 Water quality 27 Invertebrates 29 Wetland soils 30 Did you know that coal formed from swamps? 31 Wetland plants 32 Wetland fish 33 Whitebait-what are they? 34 Wetland birds 35 Food chains & food webs 37 Wetlands - a 'supermarket' for tai poutini maori 39 ACTIVITY SHEETS Activity 1: What is . a wetland? 43 Activity 2: What is . a swamp? 44 Activity 3: What is . an estuary? 45 Activity 4: What is . -

Morphological Bedload Transport in Gravel-Bed Braided Rivers
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 6-16-2017 12:00 AM Morphological Bedload Transport in Gravel-Bed Braided Rivers Sarah E. K. Peirce The University of Western Ontario Supervisor Dr. Peter Ashmore The University of Western Ontario Graduate Program in Geography A thesis submitted in partial fulfillment of the equirr ements for the degree in Doctor of Philosophy © Sarah E. K. Peirce 2017 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Physical and Environmental Geography Commons Recommended Citation Peirce, Sarah E. K., "Morphological Bedload Transport in Gravel-Bed Braided Rivers" (2017). Electronic Thesis and Dissertation Repository. 4595. https://ir.lib.uwo.ca/etd/4595 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Abstract Gravel-bed braided rivers, defined by their multi-thread planform and dynamic morphology, are commonly found in proglacial mountainous areas. With little cohesive sediment and a lack of stabilizing vegetation, the dynamic morphology of these rivers is the result of bedload transport processes. Yet, our understanding of the fundamental relationships between channel form and bedload processes in these rivers remains incomplete. For example, the area of the bed actively transporting bedload, known as the active width, is strongly linked to bedload transport rates but these relationships have not been investigated systematically in braided rivers. This research builds on previous research to investigate the relationships between morphology, bedload transport rates, and bed-material mobility using physical models of braided rivers over a range of constant channel-forming discharges and event hydrographs. -

The Major Genetic Features of the Order Odonata Are Well Known (Cf
Odonalologica 9(1): 29 JJ March /. 1980 The karyotypes of five species of Odonataendemic to New Zealand A.L. Jensen¹ Zoology Department, University of Canterbury, Christchurch-I, New Zealand Received April 10, 1979 The chromosome = 4 formula, n <5 13 (X. hi), characterises spp.: Austrolestes colensonis (White) (Lestidae), Uropetala carovei (White) (Petaluridae), Procordulia grayi (Sel.) and P. smithii (White) (Corduliidae). The karyotype of is 14 Xanthomemis zelandica (McLach.) (Coenagrionidae) described by n <J= (X). Save U. carovei of these have been far. for none spp. examined cytologically so INTRODUCTION The major genetic features of the Order Odonata are well known (cf. for example KIAUTA, 1972). The karyotypes ofalmost 500 species, representing 20 of the 27 existent families, have been determined. However, most of these records refer to Northern Hemisphere species and little cytological information is available from Australasia. Cytological studies of the many endemic species occurring in both Australia and New Zealand will therefore aid the development of a complete cytophylogenetic picture of the Order, and may also help to indicatethe relationships ofthesespecies with the world fauna. Of the II Odonata species present in New Zealand, six are endemic. of five endemic in Karyotypes the species known to occur Mid Canterbury, South Island (cf. CROSBY, DUGDALE& WATT, 1976)are presented here. MATERIAL AND METHODS and males collected Isaac's Pond Larval, teneral, mature were from (43°28’S. 172° 32'E)and/ or 0 Lake Sarah (43°03'S. 171 47’E). The testes were removed and fixed in 3; I absolutealcohohglacial 1 Present address: c o K. Andersen, Ludvig Jensensvej 3. -

Hydrodynamics of Braiding River
International Journal of Hydrology Review Article Open Access Hydrodynamics of braiding river Abstract Volume 5 Issue 3 - 2021 Braided river reaches and alluvial systems are characterized by their multi-threaded planform LUO Ching-Ruey and agents of sediment transport due to eroding and deposing to form the bars and riffles. In Associate Professor of Department of Civil Engineering, braided river, frequent sediment transport and the quick shifting of the positions about the National Chi-Nan University, Taiwan river channel induce many attentions discussion and relating a complicated consideration of the combinations of disciplines. In this article we introduce its fundamental characteristics Correspondence: LUO Ching-Ruey, Associate Professor of and further the complicated mechanism in the literature and methodologies. The braided Department of Civil Engineering, National Chi-Nan University, channel ecology and the management of braided river are mentioned and discussed, Taiwan, Email especially, the secondary currents, in this paper we explain in detail, the combinations on multiplying of 2-D flow of the velocity fluctuations. The interdisciplinary approach Received: April 28, 2021 | Published: May 10, 2021 on linking engineers, earth scientists and social scientists concerned with environmental economics, planning, and societal and political strategies in order to fully evaluate the validity and reliability of different selections to various timescales is really sensitive. Furthermore, the requirements of public education on reinforcing -

ARTHROPODA Subphylum Hexapoda Protura, Springtails, Diplura, and Insects
NINE Phylum ARTHROPODA SUBPHYLUM HEXAPODA Protura, springtails, Diplura, and insects ROD P. MACFARLANE, PETER A. MADDISON, IAN G. ANDREW, JOCELYN A. BERRY, PETER M. JOHNS, ROBERT J. B. HOARE, MARIE-CLAUDE LARIVIÈRE, PENELOPE GREENSLADE, ROSA C. HENDERSON, COURTenaY N. SMITHERS, RicarDO L. PALMA, JOHN B. WARD, ROBERT L. C. PILGRIM, DaVID R. TOWNS, IAN McLELLAN, DAVID A. J. TEULON, TERRY R. HITCHINGS, VICTOR F. EASTOP, NICHOLAS A. MARTIN, MURRAY J. FLETCHER, MARLON A. W. STUFKENS, PAMELA J. DALE, Daniel BURCKHARDT, THOMAS R. BUCKLEY, STEVEN A. TREWICK defining feature of the Hexapoda, as the name suggests, is six legs. Also, the body comprises a head, thorax, and abdomen. The number A of abdominal segments varies, however; there are only six in the Collembola (springtails), 9–12 in the Protura, and 10 in the Diplura, whereas in all other hexapods there are strictly 11. Insects are now regarded as comprising only those hexapods with 11 abdominal segments. Whereas crustaceans are the dominant group of arthropods in the sea, hexapods prevail on land, in numbers and biomass. Altogether, the Hexapoda constitutes the most diverse group of animals – the estimated number of described species worldwide is just over 900,000, with the beetles (order Coleoptera) comprising more than a third of these. Today, the Hexapoda is considered to contain four classes – the Insecta, and the Protura, Collembola, and Diplura. The latter three classes were formerly allied with the insect orders Archaeognatha (jumping bristletails) and Thysanura (silverfish) as the insect subclass Apterygota (‘wingless’). The Apterygota is now regarded as an artificial assemblage (Bitsch & Bitsch 2000).