A Taxonomic Review of the Genus Microacontias FINAL
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluating the Effectiveness of Salvage and Translocation of Striped Legless Lizards
Evaluating the effectiveness of salvage and translocation of Striped Legless Lizards Megan O’Shea February 2013 Arthur Rylah Institute for Environmental Research Technical Report Series No. 243 Evaluating the effectiveness of salvage and translocation of Striped Legless Lizards Megan O’Shea February 2013 Arthur Rylah Institute for Environmental Research Department of Sustainability and Environment Heidelberg, Victoria Report produced by: Arthur Rylah Institute for Environmental Research Department of Sustainability and Environment PO Box 137 Heidelberg, Victoria 3084 Phone (03) 9450 8600 Website: www.dse.vic.gov.au/ari © State of Victoria, Department of Sustainability and Environment 2013 This publication is copyright. Apart from fair dealing for the purposes of private study, research, criticism or review as permitted under the Copyright Act 1968 , no part may be reproduced, copied, transmitted in any form or by any means (electronic, mechanical or graphic) without the prior written permission of the State of Victoria, Department of Sustainability and Environment. All requests and enquiries should be directed to the Customer Service Centre, 136 186 or email [email protected] Citation: O’Shea, M. (2013). Evaluating the effectiveness of salvage and translocation of Striped Legless Lizards. Arthur Rylah Institute for Environmental Research Technical Report Series No. 243. Department of Sustainability and Environment, Heidelberg. ISSN 1835-3827 (print) ISSN 1835-3835 (online) ISBN 978-1-74287-763-1 (print) ISBN 978-1-74287-764-8 (online) Disclaimer: This publication may be of assistance to you but the State of Victoria and its employees do not guarantee that the publication is without flaw of any kind or is wholly appropriate for your particular purposes and therefore disclaims all liability for any error, loss or other consequence which may arise from you relying on any information in this publication. -

Terrestrial Biodiversity Compliance Report for The
TERRESTRIAL BIODIVERSITY COMPLIANCE REPORT FOR THE PROPOSED DE AAR 2 SOUTH WEF ON-SITE SUBSTATION, BATTERY ENERGY STORAGE SYSTEM (BESS) AND ANCILLARY INFRASTRUCTURE, NEAR DE AAR IN THE NORTHERN CAPE PROVINCE. For Mulilo De Aar 2 South (Pty) Ltd July 2020 Prepared By: Arcus Consultancy Services South Africa (Pty) Limited Office 607 Cube Workspace Icon Building Cnr Long Street and Hans Strijdom Avenue Cape Town 8001 T +27 (0) 21 412 1529 l E [email protected] W www.arcusconsulting.co.za Registered in South Africa No. 2015/416206/07 Terrestrial Biodiversity Compliance Report De Aar 2 South WEF Substation TABLE OF CONTENTS 1 INTRODUCTION ........................................................................................................ 3 1.1 Background .................................................................................................... 3 1.2 Scope of Study ................................................................................................ 3 1.3 Assumptions and Limitations ......................................................................... 4 2 METHODOLOGY ......................................................................................................... 4 2.1 Desk-top Study ............................................................................................... 4 2.2 Site Visit ......................................................................................................... 5 3 RESULTS AND DESCRIPTION OF THE AFFECTED ENVIRONMENT ............................ 5 3.1 Vegetation -

Reptiles and Amphibians of the Goegap Nature Reserve
their time underground in burrows. These amphibians often leave their burrows after heavy rains that are seldom. Reptiles And Amphibians Of The There are reptiles included in this report, which don’t occur here in Goegap but at the Augrabies Falls NP. So you can find here also the Nile monitor and the flat liz- Goegap Nature Reserve ard. Measuring reptiles By Tanja Mahnkopf In tortoises and terrapins the length is measured at the shell. Straight along the mid- line of the carapace. The SV-Length is the length of head and body (Snout to Vent). In lizards it easier to look for this length because their tail may be a regenerated one Introduction and these are often shorter than the original one. The length that is mentioned for the The reptiles are an ancient class on earth. The earliest reptile fossils are about 315 species in this report is the average to the maximum length. For the snakes I tried to million years old. During the aeons of time they evolved a great diversity of extinct give the total length because it is often impossible to say where the tail begins and and living reptiles. The dinosaurs and their relatives dominated the earth 150 million the body ends without holding the snake. But there was not for every snake a total years ago. Our living reptiles are remnants of that period or from a period after the length available. dinosaurs were extinct. Except of the chameleons (there are only two) you can find all reptiles in the appen- Obviously it looks like reptiles are not as successful as mammals. -

Annexure I Fauna & Flora Assessment.Pdf
Fauna and Flora Baseline Study for the De Wittekrans Project Mpumalanga, South Africa Prepared for GCS (Pty) Ltd. By Resource Management Services (REMS) P.O. Box 2228, Highlands North, 2037, South Africa [email protected] www.remans.co.za December 2008 I REMS December 2008 Fauna and Flora Assessment De Wittekrans Executive Summary Mashala Resources (Pty) Ltd is planning to develop a coal mine south of the town of Hendrina in the Mpumalanga province on a series of farms collectively known as the De Wittekrans project. In compliance with current legislation, they have embarked on the process to acquire environmental authorisation from the relevant authorities for their proposed mining activities. GCS (Pty) Ltd. commissioned Resource Management Services (REMS) to conduct a Faunal and Floral Assessment of the area to identify the potential direct and indirect impacts of future mining, to recommend management measures to minimise or prevent these impacts on these ecosystems and to highlight potential areas of conservation importance. This is the wet season survey which was done in the November 2008. The study area is located within the highveld grasslands of the Msukaligwa Local Municipality in the Gert Sibande District Municipality in Mpumalanga Province on the farms Tweefontein 203 IS (RE of Portion 1); De Wittekrans 218 IS (RE of Portion 1 and Portion 2, Portions 7, 11, 10 and 5); Groblershoek 191 IS; Groblershoop 192 IS; and Israel 207 IS. Within this region, precipitation occurs mainly in the summer months of October to March with the peak of the rainy season occurring from November to January. -

The Global Decline of Reptiles, Deja Vu Amphibians
Articles The Global Decline of Reptiles,Dé jà Vu Amphibians J. WHITFIELD GIBBONS,DAVID E. SCOTT,T R AVIS J. RYA N ,KURT A. B U H L M A N N ,T R ACEY D. TUBERV I L L E ,BRIAN S. METTS,J UDITH L. GREENE, TONY MILLS,YALE LEIDEN, SEAN POPPY,AND CHRISTOPHER T. WINNE s a group [reptiles] are neit h er ‘good ’n or ‘bad , ’ but “ ia re interes ting and unu sua l , a lt h o u gh of mi nor A i m porta n ce . If th ey should all disappe ar , it wo ul d REP TI L E SPE CI E S AR E DE CL I N IN G ON A not make mu ch difference one way or the other ”( Zim and Smith 1953,p. 9) . Fortu n a tely,this op i n i on from the Golden GLO BA L SC A L E. SI XS I G N I F IC A N T TH R E ATS Gu ide Series does not persist tod ay;most people have com e TOR E P T I L EPO P ULA T I O N SAR E H A BI T AT to recogn i ze the va lue ofboth reptiles and amphi bians as an in tegral part ofn a tu ral eco sys tems and as heralds of LO SS A N DD E G RADA TI O N , I N T RO D U C E D environ m ental qu a l ity (Gibbons and Stangel 1999). -

Flora and Fauna Specialist Assessment Report for the Proposed De Aar 2 South Grid Connection Near De Aar, Northern Cape Province
FLORA AND FAUNA SPECIALIST ASSESSMENT REPORT FOR THE PROPOSED DE AAR 2 SOUTH GRID CONNECTION NEAR DE AAR, NORTHERN CAPE PROVINCE On behalf of Mulilo De Aar 2 South (Pty) Ltd December 2020 Prepared By: Arcus Consultancy Services South Africa (Pty) Limited Office 607 Cube Workspace Icon Building Cnr Long Street and Hans Strijdom Avenue Cape Town 8001 T +27 (0) 21 412 1529 l E [email protected] W www.arcusconsulting.co.za Registered in South Africa No. 2015/416206/07 Flora & Fauna Impact Assessment Report De Aar 2 South Transmission Line and Switching Station TABLE OF CONTENTS 1 INTRODUCTION ........................................................................................................ 4 1.1 Background .................................................................................................... 4 1.2 Assessment Philosophy .................................................................................. 4 1.3 Scope of Study ................................................................................................ 5 1.4 Assumptions and Limitations ......................................................................... 5 2 METHODOLOGY ......................................................................................................... 5 3 RESULTS .................................................................................................................... 5 3.1 Vegetation ...................................................................................................... 6 3.1.1 Northern Upper -

A Phylogeny and Revised Classification of Squamata, Including 4161 Species of Lizards and Snakes
BMC Evolutionary Biology This Provisional PDF corresponds to the article as it appeared upon acceptance. Fully formatted PDF and full text (HTML) versions will be made available soon. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes BMC Evolutionary Biology 2013, 13:93 doi:10.1186/1471-2148-13-93 Robert Alexander Pyron ([email protected]) Frank T Burbrink ([email protected]) John J Wiens ([email protected]) ISSN 1471-2148 Article type Research article Submission date 30 January 2013 Acceptance date 19 March 2013 Publication date 29 April 2013 Article URL http://www.biomedcentral.com/1471-2148/13/93 Like all articles in BMC journals, this peer-reviewed article can be downloaded, printed and distributed freely for any purposes (see copyright notice below). Articles in BMC journals are listed in PubMed and archived at PubMed Central. For information about publishing your research in BMC journals or any BioMed Central journal, go to http://www.biomedcentral.com/info/authors/ © 2013 Pyron et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes Robert Alexander Pyron 1* * Corresponding author Email: [email protected] Frank T Burbrink 2,3 Email: [email protected] John J Wiens 4 Email: [email protected] 1 Department of Biological Sciences, The George Washington University, 2023 G St. -

Khutala 5-Seam Mining Project: Mpumalanga Province Terrestrial
Author: Barbara Kasl E-mail: [email protected] Khutala 5-Seam Mining Project: Mpumalanga Province Terrestrial Fauna Biodiversity Impact Assessment May 2021 Copyright is the exclusive property of the author. All rights reserved. This report or any portion thereof may not be reproduced or used in any manner whatsoever without the express permission of the author except for the use of quotations properly cited and referenced. This document may not be modified other than by the author. Khutala 5-Seam Mining Project: Terrestrial Fauna Impact Assessment Report May 2021 Specialist Qualification & Declaration Barbara Kasl (CV summary attached as Appendix A): • Holds a PhD in Animal, Plant and Environmental Sciences from the University of the Witwatersrand; • Is a registered SACNASP Professional Ecological and Environmental Scientist (Pr.Sci.Nat. Registration No.: 400257/09), with expertise in faunal ecology; and • Has been actively involved in the environmental consultancy field for over 13 years. I, Barbara Kasl, confirm that: • I act as independent consultant and specialist in the field of ecology and environmental sciences; • I have no vested interest in the project other than remuneration for work completed in terms of the Scope of Work; • I have presented the information in this report in line with the requirements of the Animal Species and Terrestrial Biodiversity Protocols as required under the National Environmental Management Act (107/1998) (NEMA) as far as these are relevant to the specific Scope of Work; • I have taken NEMA Principals into account as far as these are relevant to the Scope of Work; and • Information presented is, to the best of my knowledge, accurate and correct within the restraints of stipulated limitations. -
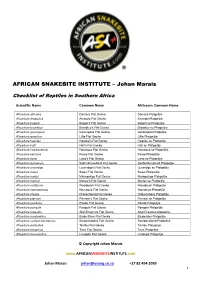
Johan Marais
AFRICAN SNAKEBITE INSTITUTE – Johan Marais Checklist of Reptiles in Southern Africa Scientific Name Common Name Afrikaans Common Name Afroedura africana Damara Flat Gecko Damara Platgeitjie Afroedura amatolica Amatola Flat Gecko Amatola Platgeitjie Afroedura bogerti Bogert's Flat Gecko Bogert se Platgeitjie Afroedura broadleyi Broadley’s Flat Gecko Broadley se Platgeitjie Afroedura gorongosa Gorongosa Flat Gecko Gorongosa Platgeitjie Afroedura granitica Lillie Flat Gecko Lillie Platgeitjie Afroedura haackei Haacke's Flat Gecko Haacke se Platgeitjie Afroedura halli Hall's Flat Gecko Hall se Platgeitjie Afroedura hawequensis Hawequa Flat Gecko Hawequa se Platgeitjie Afroedura karroica Karoo Flat Gecko Karoo Platgeitjie Afroedura langi Lang's Flat Gecko Lang se Platgeitjie Afroedura leoloensis Sekhukhuneland Flat Gecko Sekhukhuneland Platgeitjie Afroedura loveridgei Loveridge's Flat Gecko Loveridge se Platgeitjie Afroedura major Swazi Flat Gecko Swazi Platgeitjie Afroedura maripi Mariepskop Flat Gecko Mariepskop Platgeitjie Afroedura marleyi Marley's Flat Gecko Marley se Platgeitjie Afroedura multiporis Woodbush Flat Gecko Woodbush Platgeijtie Afroedura namaquensis Namaqua Flat Gecko Namakwa Platgeitjie Afroedura nivaria Drakensberg Flat Gecko Drakensberg Platgeitjie Afroedura pienaari Pienaar’s Flat Gecko Pienaar se Platgeitjie Afroedura pondolia Pondo Flat Gecko Pondo Platgeitjie Afroedura pongola Pongola Flat Gecko Pongola Platgeitjie Afroedura rupestris Abel Erasmus Flat Gecko Abel Erasmus platgeitjie Afroedura rondavelica Blyde River -

Patterns of Species Richness, Endemism and Environmental Gradients of African Reptiles
Journal of Biogeography (J. Biogeogr.) (2016) ORIGINAL Patterns of species richness, endemism ARTICLE and environmental gradients of African reptiles Amir Lewin1*, Anat Feldman1, Aaron M. Bauer2, Jonathan Belmaker1, Donald G. Broadley3†, Laurent Chirio4, Yuval Itescu1, Matthew LeBreton5, Erez Maza1, Danny Meirte6, Zoltan T. Nagy7, Maria Novosolov1, Uri Roll8, 1 9 1 1 Oliver Tallowin , Jean-Francßois Trape , Enav Vidan and Shai Meiri 1Department of Zoology, Tel Aviv University, ABSTRACT 6997801 Tel Aviv, Israel, 2Department of Aim To map and assess the richness patterns of reptiles (and included groups: Biology, Villanova University, Villanova PA 3 amphisbaenians, crocodiles, lizards, snakes and turtles) in Africa, quantify the 19085, USA, Natural History Museum of Zimbabwe, PO Box 240, Bulawayo, overlap in species richness of reptiles (and included groups) with the other ter- Zimbabwe, 4Museum National d’Histoire restrial vertebrate classes, investigate the environmental correlates underlying Naturelle, Department Systematique et these patterns, and evaluate the role of range size on richness patterns. Evolution (Reptiles), ISYEB (Institut Location Africa. Systematique, Evolution, Biodiversite, UMR 7205 CNRS/EPHE/MNHN), Paris, France, Methods We assembled a data set of distributions of all African reptile spe- 5Mosaic, (Environment, Health, Data, cies. We tested the spatial congruence of reptile richness with that of amphib- Technology), BP 35322 Yaounde, Cameroon, ians, birds and mammals. We further tested the relative importance of 6Department of African Biology, Royal temperature, precipitation, elevation range and net primary productivity for Museum for Central Africa, 3080 Tervuren, species richness over two spatial scales (ecoregions and 1° grids). We arranged Belgium, 7Royal Belgian Institute of Natural reptile and vertebrate groups into range-size quartiles in order to evaluate the Sciences, OD Taxonomy and Phylogeny, role of range size in producing richness patterns. -

New Zealand Threat Classification System (NZTCS)
NEW ZEALAND THREAT CLASSIFICATION SERIES 17 Conservation status of New Zealand reptiles, 2015 Rod Hitchmough, Ben Barr, Marieke Lettink, Jo Monks, James Reardon, Mandy Tocher, Dylan van Winkel and Jeremy Rolfe Each NZTCS report forms part of a 5-yearly cycle of assessments, with most groups assessed once per cycle. This report is the first of the 2015–2020 cycle. Cover: Cobble skink, Oligosoma aff.infrapunctatum “cobble”. Photo: Tony Jewell. New Zealand Threat Classification Series is a scientific monograph series presenting publications related to the New Zealand Threat Classification System (NZTCS). Most will be lists providing NZTCS status of members of a plant or animal group (e.g. algae, birds, spiders). There are currently 23 groups, each assessed once every 3 years. After each three-year cycle there will be a report analysing and summarising trends across all groups for that listing cycle. From time to time the manual that defines the categories, criteria and process for the NZTCS will be reviewed. Publications in this series are considered part of the formal international scientific literature. This report is available from the departmental website in pdf form. Titles are listed in our catalogue on the website, refer www.doc.govt.nz under Publications, then Series. © Copyright December 2016, New Zealand Department of Conservation ISSN 2324–1713 (web PDF) ISBN 978–1–98–851400–0 (web PDF) This report was prepared for publication by the Publishing Team; editing and layout by Lynette Clelland. Publication was approved by the Director, Terrestrial Ecosystems Unit, Department of Conservation, Wellington, New Zealand. Published by Publishing Team, Department of Conservation, PO Box 10420, The Terrace, Wellington 6143, New Zealand. -

The Distribution and Abundance of Herpetofauna on a Quaternary Aeolian Dune Deposit: Implications for Strip Mining
The distribution and abundance of herpetofauna on a Quaternary aeolian dune deposit: Implications for Strip Mining Bryan Maritz A dissertation submitted to the School of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, South Africa in fulfilment of the requirements of the degree of Masters of Science. Johannesburg, South Africa July, 2007 ABSTRACT Exxaro KZN Sands is planning the development of a heavy minerals strip mine south of Mtunzini, KwaZulu-Natal, South Africa. The degree to which mining activities will affect local herpetofauna is poorly understood and baseline herpetofaunal diversity data are sparse. This study uses several methods to better understand the distribution and abundance of herpetofauna in the area. I reviewed the literature for the grid squares 2831DC and 2831 DD and surveyed for herpetofauna at the study site using several methods. I estimate that 41 amphibian and 51 reptile species occur in these grid squares. Of these species, 19 amphibian and 39 reptile species were confirmed for the study area. In all, 29 new unique, grid square records were collected. The paucity of ecological data for cryptic fauna such as herpetofauna is particularly evident for taxa that are difficult to sample. Because fossorial herpetofauna spend most of their time below the ground surface, their ecology and biology are poorly understood and warrant further investigation. I sampled fossorial herpetofauna using two excavation techniques. Sites were selected randomly from the study area which was expected to host high fossorial herpetofaunal diversity and abundance. A total of 218.6 m3 of soil from 311 m2 (approximately 360 metric tons) was excavated and screened for herpetofauna.