Androgen and Estrogen Receptor Expression in the Developing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reference Sheet 1
MALE SEXUAL SYSTEM 8 7 8 OJ 7 .£l"00\.....• ;:; ::>0\~ <Il '"~IQ)I"->. ~cru::>s ~ 6 5 bladder penis prostate gland 4 scrotum seminal vesicle testicle urethra vas deferens FEMALE SEXUAL SYSTEM 2 1 8 " \ 5 ... - ... j 4 labia \ ""\ bladderFallopian"k. "'"f"";".'''¥'&.tube\'WIT / I cervixt r r' \ \ clitorisurethrauterus 7 \ ~~ ;~f4f~ ~:iJ 3 ovaryvagina / ~ 2 / \ \\"- 9 6 adapted from F.L.A.S.H. Reproductive System Reference Sheet 3: GLOSSARY Anus – The opening in the buttocks from which bowel movements come when a person goes to the bathroom. It is part of the digestive system; it gets rid of body wastes. Buttocks – The medical word for a person’s “bottom” or “rear end.” Cervix – The opening of the uterus into the vagina. Circumcision – An operation to remove the foreskin from the penis. Cowper’s Glands – Glands on either side of the urethra that make a discharge which lines the urethra when a man gets an erection, making it less acid-like to protect the sperm. Clitoris – The part of the female genitals that’s full of nerves and becomes erect. It has a glans and a shaft like the penis, but only its glans is on the out side of the body, and it’s much smaller. Discharge – Liquid. Urine and semen are kinds of discharge, but the word is usually used to describe either the normal wetness of the vagina or the abnormal wetness that may come from an infection in the penis or vagina. Duct – Tube, the fallopian tubes may be called oviducts, because they are the path for an ovum. -

Analysis of Estrogen Receptor Isoforms and Variants in Breast Cancer Cell Lines
EXPERIMENTAL AND THERAPEUTIC MeDICINE 2: 537-544, 2011 Analysis of estrogen receptor isoforms and variants in breast cancer cell lines MAIE AL-BADER1, CHRISTOPHER FORD2, BUSHRA AL-AYADHY3 and ISSAM FRANCIS3 Departments of 1Physiology, 2Surgery, and 3Pathology, Faculty of Medicine, Kuwait University, Safat 13110, Kuwait Received November 22, 2010; Accepted February 14, 2011 DOI: 10.3892/etm.2011.226 Abstract. In the present study, the expression of estrogen domain C, the DNA binding domain; domains D/E, bearing receptor (ER)α and ERβ isoforms in ER-positive (MCF7, both the activation function-2 (AF-2) and the ligand binding T-47D and ZR-75-1) and ER-negative (MDA-MB-231, SK-BR-3, domains; and finally, domain F, the C-terminal domain (6,7). MDA-MB-453 and HCC1954) breast cancer cell lines was The actions of estrogens are mediated by binding to ERs investigated. ERα mRNA was expressed in ER-positive and (ERα and/or ERβ). These receptors, which are co-expressed some ER-negative cell lines. ERα ∆3, ∆5 and ∆7 spliced in a number of tissues, form functional homodimers or variants were present in MCF7 and T-47D cells; ERα ∆5 heterodimers. When bound to estrogens as homodimers, the and ∆7 spliced variants were detected in ZR-75-1 cells. transcription of target genes is activated (8,9), while as heterodi- MDA-MB-231 and HCC1954 cells expressed ERα ∆5 and ∆7 mers, ERβ exhibits an inhibitory action on ERα-mediated gene spliced variants. The ERβ1 variant was expressed in all of the expression and, in many instances, opposes the actions of ERα cell lines and the ERβ2 variant in all of the ER-positive and (7,9). -

The Evolutionary History of the Human Face
This is a repository copy of The evolutionary history of the human face. White Rose Research Online URL for this paper: https://eprints.whiterose.ac.uk/145560/ Version: Accepted Version Article: Lacruz, Rodrigo S, Stringer, Chris B, Kimbel, William H et al. (5 more authors) (2019) The evolutionary history of the human face. Nature Ecology and Evolution. pp. 726-736. ISSN 2397-334X https://doi.org/10.1038/s41559-019-0865-7 Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ THE EVOLUTIONARY HISTORY OF THE HUMAN FACE Rodrigo S. Lacruz1*, Chris B. Stringer2, William H. Kimbel3, Bernard Wood4, Katerina Harvati5, Paul O’Higgins6, Timothy G. Bromage7, Juan-Luis Arsuaga8 1* Department of Basic Science and Craniofacial Biology, New York University College of Dentistry; and NYCEP, New York, USA. 2 Department of Earth Sciences, Natural History Museum, London, UK 3 Institute of Human Origins and School of Human Evolution and Social Change, Arizona State University, Tempe, AZ. -

Expression of Aromatase, Estrogen Receptor and , Androgen
0031-3998/06/6006-0740 PEDIATRIC RESEARCH Vol. 60, No. 6, 2006 Copyright © 2006 International Pediatric Research Foundation, Inc. Printed in U.S.A. Expression of Aromatase, Estrogen Receptor ␣ and , Androgen Receptor, and Cytochrome P-450scc in the Human Early Prepubertal Testis ESPERANZA B. BERENSZTEIN, MARI´A SONIA BAQUEDANO, CANDELA R. GONZALEZ, NORA I. SARACO, JORGE RODRIGUEZ, ROBERTO PONZIO, MARCO A. RIVAROLA, AND ALICIA BELGOROSKY Research Laboratory [E.B.B., M.S.B., C.R.G., N.I.S., J.R., M.A.R., A.B.], Hospital de Pediatria Garrahan, Buenos Aires C124 5AAM, Argentina; Centro de Investigaciones en Reproduccion [R.P.], Facultad de Medicina, Universidad de Buenos Aires, Buenos Aires C112 1ABG, Argentina ABSTRACT: The expression of aromatase, estrogen receptor ␣ might affect adult testicular cell mass, as well as testicular (ER␣) and  (ER), androgen receptor (AR), and cytochrome P-450 function (8). side chain cleavage enzyme (cP450scc) was studied in prepubertal In humans (9), there are three growth phases of LCs testis. Samples were divided in three age groups (GRs): GR1, during testicular development. Fetal LCs produce testoster- ϭ newborns (1- to 21-d-old neonates, n 5); GR2, postnatal activation one required for fetal masculinization and Insl-3, necessary ϭ stage (1- to 7-mo-old infants, n 6); GR3, childhood (12- to for testicular descent (10). They regress during the third ϭ ␣ 60-mo-old boys, n 4). Absent or very poor detection of ER by trimester of pregnancy. A second wave of infantile LCs has immunohistochemistry in all cells and by mRNA expression was been described during the postnatal surge of luteinizing observed. -
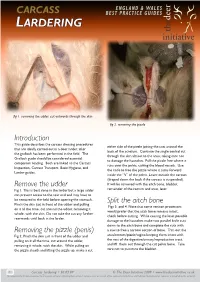
Introduction Remove the Udder Removing the Pizzle (Penis)
fig . removing the udder, cut outwards through the skin fig 2. removing the pizzle Introduction This guide describes the carcass dressing procedures either side of the pizzle joining the cuts around the that are ideally carried out in a deer larder, after back of the scrotum. Continue the single central cut the gralloch has been performed in the field. The through the skin almost to the anus, taking care not Gralloch guide should be considered essential to damage the haunches. Pull the pizzle free where it companion reading. Both are linked to the Carcass runs over the pelvis, cutting the blood vessels. Use Inspection, Carcass Transport, Basic Hygiene, and the knife to free the pizzle where it turns forward Larder guides. inside the “V” of the pelvis. Leave outside the carcass (draped down the back if the carcass is suspended). Remove the udder It will be removed with the aitch bone, bladder, Fig 1. This is best done in the larder but a large udder remainder of the rectum and anus, later. can prevent access to the rear end and may have to be removed in the field before opening the stomach. Split the aitch bone Pinch the skin just in front of the udder and pulling Figs 3. and 4. Note that some venison processors on it all the time, cut around the udder, removing it would prefer that the aitch bone remains intact, whole, with the skin. Do not take the cut any further check before cutting. While causing the least possible rearwards until back in the larder. -

Effects of Glans Penis Augmentation Using Hyaluronic Acid Gel for Premature Ejaculation
International Journal of Impotence Research (2004) 16, 547–551 & 2004 Nature Publishing Group All rights reserved 0955-9930/04 $30.00 www.nature.com/ijir Effects of glans penis augmentation using hyaluronic acid gel for premature ejaculation JJ Kim1, TI Kwak1, BG Jeon1, J Cheon1 and DG Moon1* 1Department of Urology, Korea University College of Medicine, Sungbuk-ku, Seoul, Korea The main limitation of medical treatment for premature ejaculation is recurrence after withdrawal of medication. We evaluated the effect of glans penis augmentation using injectable hyaluronic acid (HA) gel for the treatment of premature ejaculation via blocking accessibility of tactile stimuli to nerve receptors. In 139 patients of premature ejaculation, dorsal neurectomy (Group I, n ¼ 25), dorsal neurectomy with glandular augmentation (Group II, n ¼ 49) and glandular augmentation (Group III, n ¼ 65) were carried out, respectively. Two branches of dorsal nerve preserving that of midline were cut at 2 cm proximal to coronal sulcus. For glandular augmentation, 2 cc of HA was injected into the glans penis, subcutaneously. At 6 months after each procedure, changes of glandular circumference were measured by tapeline in Groups II and III. In each groups, ejaculation time, patient’s satisfaction and partner’s satisfaction were also assessed. There was no significant difference in preoperative ejaculation time among three groups. Preoperative ejaculation times were 89.2740.29, 101.54759.42 and 96.5752.32 s in Groups I, II and III, respectively. Postoperative ejaculation times were significantly increased to 235.6758.6, 324.247107.58 and 281.9793.2 s in Groups I, II and III, respectively (Po0.01). -

Standard Human Facial Proportions
Name:_____________________________________________ Date:__________________Period: __________________ Standard Human Facial Proportions: The standard proportions for the human head can help you place facial features and find their orientation. The list below gives an idea of ideal proportions. • The eyes are halfway between the top of the head and the chin. • The face is divided into 3 parts from the hairline to the eyebrow, from the eyebrow to the bottom of the nose, and from the nose to the chin. • The bottom of the nose is halfway between the eyes and the chin. • The mouth is one third of the distance between the nose and the chin. • The distance between the eyes is equal to the width of one eye. • The face is about the width of five eyes and about the height of about seven eyes. • The base of the nose is about the width of the eye. • The mouth at rest is about the width of an eye. • The corners of the mouth line up with the centers of the eye. Their width is the distance between the pupils of the eye. • The top of the ears line up slightly above the eyes in line with the outer tips of the eyebrows. • The bottom of the ears line up with the bottom of the nose. • The width of the shoulders is equal to two head lengths. • The width of the neck is about ½ a head. Facial Feature Examples.docx Page 1 of 13 Name:_____________________________________________ Date:__________________Period: __________________ PROFILE FACIAL PROPORTIONS Facial Feature Examples.docx Page 2 of 13 Name:_____________________________________________ Date:__________________Period: -

How to Create the Perfect Eyebrow
Creating the Perfect Brows . As a make-up artist, I was acutely aware of the fact that my mother had a need that conventional cosmetics could not meet. She suffered from a type of alopecia (hair loss) of the eyebrows and lashes ever since I could remember. I would draw the eyebrows on for her, but it was often a painstaking process for her to draw the eyebrows on herself on a daily basis. After my mom described the embarrassment of swimming and having the pencil wash off her eyebrows, and another afternoon when a neighbor stopped by after she had been napping and one eyebrow was completely wiped off when she answered the door, it clearly was time for a solution. After completing my certification with the Academy of Micropigmentation, I had one of the most fulfilling days of my life, the day I was able to give a gift of eyebrows to my mother. This was a life changing experience for her and an incredible boost to her self-image. She went on to do her eyeliner, which was especially beneficial to her due to the absence of eyelashes, and I was able to give her lips a fuller appearance with the permanent lipliner. It is said that the facial feature models spend the most time on is their eyebrows and most women, at some point, have altered their eyebrows by tweezing, waxing or electrolysis. As women grow older, the hair on the outer half of their brows tend to become sparse or even non-existent. In ancient China, royalty were the only ones adorned with “tattooed” eyebrows. -

Expression of Estrogen Receptor-Beta Isoforms in Barrett's
ANTICANCER RESEARCH 24: 2919-2924 (2004) Expression of Estrogen Receptor-Beta Isoforms in Barrett’s Metaplasia, Dysplasia and Esophageal Adenocarcinoma LIANG LIU, MINNI CHIRALA and MAMOUN YOUNES Departments of Pathology, Baylor College of Medicine and The Methodist Hospital, Houston, TX 77030, U.S.A. Abstract. We have previously shown that the majority of and beta (ER-B). ER-A is mainly expressed in female sex esophageal adenocarcinomas (EA), and its precursor Barrett’s organs, such as breast and uterus (2), whereas ER-B is metaplasia (BM), express estrogen receptor beta (ER-B). Several expressed in both sex organs and other tissues, such as isoforms of ER-B have been described and are presumed to have prostate, lung, thyroid, adrenal cortex and testis (3). Several different functions, but their distribution in BM and EA is not ER-B isoforms have been identified and characterized in known. The aim of this work was to determine which ER-B many non-gynecologic tumors including carcinomas of the isoforms are expressed in EA and BM. Sections of formalin-fixed lung (4), prostate (5), colon (6, 7) and stomach (8). and paraffin-embedded esophageal tissue from 33 esophageactomy Tamoxifen, a specific ER-B antagonist, has been successfully specimens, of which 27 had invasive EA, were stained for the ER- used in the treatment of patients with breast carcinoma B isoforms ER-B1, ER-B2, ER-B3 and ER-B5 utilizing the (9,10) and it has been shown that ER-B status is a significant immunoperoxidase method. ER-B1 was detected in 23 out of 27 predictor of survival in women with breast carcinoma treated (85%) EA compared to 3 out of 14 (21%) Barrett’s metaplasia with mastectomy and adjuvant tamoxifen (11). -

Influence of Androgen Receptor on the Prognosis of Breast Cancer
Journal of Clinical Medicine Article Influence of Androgen Receptor on the Prognosis of Breast Cancer 1, , 2, 1 2 3 Ki-Tae Hwang * y , Young A Kim y , Jongjin Kim , Jeong Hwan Park , In Sil Choi , Kyu Ri Hwang 4 , Young Jun Chai 1 and Jin Hyun Park 3 1 Department of Surgery, Seoul Metropolitan Government Seoul National University Boramae Medical Center, 39, Boramae-Gil, Dongjak-gu, Seoul 156-707, Korea; [email protected] (J.K.); [email protected] (Y.J.C.) 2 Department of Pathology, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul 156-707, Korea; [email protected] (Y.A.K.); [email protected] (J.H.P.) 3 Department of Internal Medicine, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul 156-707, Korea; [email protected] (I.S.C.); [email protected] (J.H.P.) 4 Department of Obstetrics & Gynecology, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Seoul 156-707, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-2-870-2275; Fax: +82-2-831-2826 These authors contributed equally to this work. y Received: 28 February 2020; Accepted: 8 April 2020; Published: 10 April 2020 Abstract: We investigated the prognostic influence of androgen receptor (AR) on breast cancer. AR status was assessed using immunohistochemistry with tissue microarrays from 395 operable primary breast cancer patients who received curative surgery. The Kaplan–Meier estimator was used to analyze the survival rates and a log-rank test was used to determine the significance of the differences in survival. The Cox proportional hazards model was used to calculate the hazard ratio (HR) and the 95% confidence interval (CI) of survival. -

View of Urothelial and Metastatic Carcinoma Including Clinical Presentation, Diagnostic Testing, Treatment and Chiropractic Considerations Is Discussed
Daniels et al. Chiropractic & Manual Therapies (2016) 24:14 DOI 10.1186/s12998-016-0097-8 CASE REPORT Open Access Bladder metastasis presenting as neck, arm and thorax pain: a case report Clinton J. Daniels1,2,3*, Pamela J. Wakefield1,2 and Glenn A. Bub1,2 Abstract Background: A case of metastatic carcinoma secondary to urothelial carcinoma presenting as musculoskeletal pain is reported. A brief review of urothelial and metastatic carcinoma including clinical presentation, diagnostic testing, treatment and chiropractic considerations is discussed. Case presentation: This patient presented in November 2014 with progressive neck, thorax and upper extremity pain. Computed tomography revealed a destructive soft tissue mass in the cervical spine and additional lytic lesion of the 1st rib. Prompt referral was made for surgical consultation and medical management. Conclusion: Distant metastasis is rare, but can present as a musculoskeletal complaint. History of carcinoma should alert the treating chiropractic physician to potential for serious disease processes. Keywords: Chiropractic, Neck pain, Transitional cell carcinoma, Bladder cancer, Metastasis, Case report Background serious complication of UC is distant metastasis—with Urothelial carcinoma (UC), also known as transitional higher stage cancer and lymph involvement worsening cell carcinoma (TCC), accounts for more than 90 % of prognosis and cancer survival rate [10]. The 5-year all bladder cancers and commonly metastasizes to the cancer-specific survival rate of UC is estimated to be pelvic lymph nodes, lungs, liver, bones and adrenals or 78 % [10, 11]. brain [1, 2]. The spread of bladder cancer is mainly done Neck pain accounts for 24 % of all disorders seen by via the lymphatic system with the most frequent location chiropractors [12]. -

Estrogen-Related Receptor Alpha: an Under-Appreciated Potential Target for the Treatment of Metabolic Diseases
International Journal of Molecular Sciences Review Estrogen-Related Receptor Alpha: An Under-Appreciated Potential Target for the Treatment of Metabolic Diseases Madhulika Tripathi, Paul Michael Yen and Brijesh Kumar Singh * Laboratory of Hormonal Regulation, Cardiovascular and Metabolic Disorders Program, Duke-NUS Medical School, Singapore 169857, Singapore; [email protected] (M.T.); [email protected] (P.M.Y.) * Correspondence: [email protected] Received: 7 February 2020; Accepted: 24 February 2020; Published: 28 February 2020 Abstract: The estrogen-related receptor alpha (ESRRA) is an orphan nuclear receptor (NR) that significantly influences cellular metabolism. ESRRA is predominantly expressed in metabolically-active tissues and regulates the transcription of metabolic genes, including those involved in mitochondrial turnover and autophagy. Although ESRRA activity is well-characterized in several types of cancer, recent reports suggest that it also has an important role in metabolic diseases. This minireview focuses on the regulation of cellular metabolism and function by ESRRA and its potential as a target for the treatment of metabolic disorders. Keywords: estrogen-related receptor alpha; mitophagy; mitochondrial turnover; metabolic diseases; non-alcoholic fatty liver disease (NAFLD); adipogenesis; adaptive thermogenesis 1. Introduction When the estrogen-related receptor alpha (ESRRA) was first cloned, it was found to be a nuclear receptor (NR) that had DNA sequence homology to the estrogen receptor alpha (ESR1) [1]. There are several examples of estrogen-related receptor (ESRR) and estrogen-signaling cross-talk via mutual transcriptional regulation or reciprocal binding to each other’s response elements of common target genes in a context-specific manner [2,3].