FUSF Symposium Program and Abstract Book 2014 (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An International Journal for Students of Theological and Religious Studies Volume 36 Issue 2 July 2011
An International Journal for Students of Theological and Religious Studies Volume 36 Issue 2 July 2011 EDITORIAL: Generational Conflict in Ministry 180 D. A. Carson MINORITY REPORT: A Word to the Conscience 183 Carl Trueman Is the Reformation Over? John Calvin, Roman Catholicism, 185 and Contemporary Ecumenical Conversations Scott M. Manetsch Intrinsic Canonicity and the Inadequacy of the 203 Community Approach to Canon-Determination John C. Peckham Canon as Tradition: The New Covenant and the 216 Hermeneutical Question Mark R. Saucy Not Ashamed! The Sufficiency of Scripture for 238 Public Theology Dan Strange A Preacher’s Decalogue 261 Sinclair B. Ferguson Book Reviews 269 DESCRIPTION Themelios is an international evangelical theological journal that expounds and defends the historic Christian faith. Its primary audience is theological students and pastors, though scholars read it as well. It was formerly a print journal operated by RTSF/UCCF in the UK, and it became a digital journal operated by The Gospel Coalition in 2008. The new editorial team seeks to preserve representation, in both essayists and reviewers, from both sides of the Atlantic. Themelios is published three times a year exclusively online at www.theGospelCoalition.org. It is presented in two formats: PDF (for citing pagination) and HTML (for greater accessibility, usability, and infiltration in search engines). Themelios is copyrighted by The Gospel Coalition. Readers are free to use it and circulate it in digital form without further permission (any print use requires further written permission), but they must acknowledge the source and, of course, not change the content. EDITORS BOOK ReVIEW EDITORS Systematic Theology and Bioethics Hans Madueme General Editor: D. -

Sessions Full Week
MONDAY MORNING, 2 DECEMBER 2013 GOLDEN GATE 4/5, 9:00 A.M. TO 11:45 A.M Session 1aAA Architectural Acoustics: General Topics in Architectural Acoustics 1a MON. AM Steven D. Pettyjohn, Chair The Acoustics & Vibration Group, Inc., 5700 Broadway, Sacramento, CA 95820 Contributed Papers 9:00 mechanisms allows us to predict how this ability is lost in the presence of reflections and noise, and to predict a number of ways that real clarity can 1aAA1. Toward reliable metrics for Sacred Harp singing spaces. be measured and optimized in classrooms, lecture halls, and performance Benjamin J. Copenhaver, Scott J. Schoen, and Michael R. Haberman venues of all types. This paper will describe and demonstrate how reflec- (Mech. Eng. Dept. and Appl. Res. Labs., The Univ. of Texas at Austin, P.O. tions degrade the closeness or clarity of sounds, and how this degradation Box 8029, Austin, TX 78713-8029, [email protected]) can be prevented or ameliorated. Examples of old and new spaces with ei- Sacred Harp singing, a common type of shape-note singing, is a centu- ther excellent or poor clarity will be presented, along with a few examples ries-old tradition of American community choral music. It is traditionally a of recent improvements to existing halls. participatory form of music with no distinction between performers and au- dience, a characteristic that makes for acoustical requirements that differ 09:45 considerably from those of a concert hall or even a typical worship space. In the spirit of the text Concert Halls and Opera Houses by L. Beranek, we 1aAA4. -

MULLERES CON CIENCIA NA HISTORIA Manol@ R
MULLERES CON CIENCIA NA HISTORIA Manol@ R. Bermejo,a Ana González-Noya,a Rosa Pedrido,a Mª José Romero,a Lucía González-Louro,a Carmen Romero,a M. Inés García-Seijo,b Isabel Fernández,a Esther Gómez-Fórneas,a Beatriz Fernández,a Mª Jesús Rodríguez,a María A. Lires c. aUniversidade de Santiago de Compostela; bIES Monte Castelo (Burela); cUniversidade de Vigo 1.- Introducción Cada vez é máis importante, e necesaria, a incorporación da historia da ciencia e do pensamento científico á Didáctica da aula. Temos que adeprender a: contextualizar os descubrimentos científicos e as aportacións tecnolóxicas; presentar os saltos cualitativos de pensamento; indicar os persoeiros e persoeiras máis sobranceiros no campo das ciencias; ........, etc. Os nosos alumnos deberán comprender que: O desenvolvemento científico/tecnolóxico actual non é froito da casualidade; que tódolos descubrimentos non se fixeron nunha determinada época da historia; que a ciencia non ten xénero, aínda que estén seguros de que a ciencia é masculina porque o din os libros; que a ciencia é máis un constructo colectivo que o resultado de aportacións individuais; ..........., etc. Na liña de defender que a muller está na Ciencia desde sempre; que, dende sempre, ven facendo aportacións ó descubrimento científico; que a construcción do pensamento científico corresponde tanto á muller coma ó home, un grupo de profesoras de ENCIGA adicámonos a traballar sobre o tema do xénero na Ciencia. Presentamos, ó longo dos últimos anos, varias comunicacións a congresos de ENCIGA (1, 2); abrimos unha sección sobre o xénero na ciencia no noso Boletín (3, 4, 5) na que postulábamos quen podería ter sido a descubridora de: O calendario (3); a metalurxia (4); a aspirina (5); escribimos libros (6) e materiais didácticos, ........., etc. -

VENUS Corona M N R S a Ak O Ons D M L YN a G Okosha IB E .RITA N Axw E a I O
N N 80° 80° 80° 80° L Dennitsa D. S Yu O Bachue N Szé K my U Corona EG V-1 lan L n- H V-1 Anahit UR IA ya D E U I OCHK LANIT o N dy ME Corona A P rsa O r TI Pomona VA D S R T or EG Corona E s enpet IO Feronia TH L a R s A u DE on U .TÜN M Corona .IV Fr S Earhart k L allo K e R a s 60° V-6 M A y R 60° 60° E e Th 60° N es ja V G Corona u Mon O E Otau nt R Allat -3 IO l m k i p .MARGIT M o E Dors -3 Vacuna Melia o e t a M .WANDA M T a V a D o V-6 OS Corona na I S H TA R VENUS Corona M n r s a Ak o ons D M L YN A g okosha IB E .RITA n axw e A I o U RE t M l RA R T Fakahotu r Mons e l D GI SSE I s V S L D a O s E A M T E K A N Corona o SHM CLEOPATRA TUN U WENUS N I V R P o i N L I FO A A ght r P n A MOIRA e LA L in s C g M N N t K a a TESSERA s U . P or le P Hemera Dorsa IT t M 11 km e am A VÉNUSZ w VENERA w VENUE on Iris DorsaBARSOVA E I a E a A s RM A a a OLO A R KOIDULA n V-7 s ri V VA SSE e -4 d E t V-2 Hiei Chu R Demeter Beiwe n Skadi Mons e D V-5 S T R o a o r LI s I o R M r Patera A I u u s s V Corona p Dan o a s Corona F e A o A s e N A i P T s t G yr A A i U alk 1 : 45 000 000 K L r V E A L D DEKEN t Baba-Jaga D T N T A a PIONEER or E Aspasia A o M e s S a (1 MM= 45 KM) S r U R a ER s o CLOTHO a A N u s Corona a n 40° p Neago VENUS s s 40° s 40° o TESSERA r 40° e I F et s o COCHRAN ZVEREVA Fluctus NORTH 0 500 1000 1500 2000 2500 KM A Izumi T Sekhm n I D . -

NAMED VENUSIAN CRATERS; Joel F
NAMED VENUSIAN CRATERS; Joel F. Russell and Gerald G. Schaber, U.S. Geological Survey, 2255 N. Gemini Dr. Flagstaff, AZ 86001 Schaber et al. [I] compiled a database of 841 craters on Venus, based on Magellan coverage of 89% of the planet's surface. That database, derived from coverage of approximately 98% of Venus' surface, has been expanded to 912 craters, ranging in diameter from 1.5 to 280 krn [2]. About 150 of the larger craters were previously identified by Pioneer Venus and Soviet Venera projects and subsequently forrnally named by the International Astronomical Union (IAU). A few of the features identified and nanled as impact craters on Pioneer and Venera images have not been recognized on Magellan images, and therefore the IAU is being requested to drop their names. For example, the feature known as Cleopatra is officially named as a patera, although it is now generally accepted that Cleopatra is a crater [I]. Also, the feature Eve, which has been used to define the prime meridian for Venus, was erroneously identified as an impact feature, but its true morphology has not been determined from Magellan images. The Magellan project has requested the IAU to name hundreds of craters identified by Magellan. At its triennial General Assembly in Buenos Aires in 1991, the IAU [3] gave full approval to names for 102 craters (table 1) in addition to those previously named. At its 1992 meeting, the IAU's Working Group for Planetary System Nomenclature, which screens all planetary names prior to formal consideration by the General Assembly, gave provisional approval to names for an additional 239 Venusian craters. -
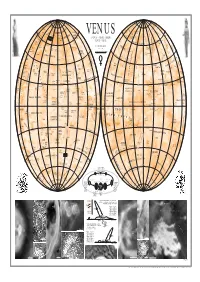
1 : 45 000 000 E a CORONA D T N O M E Or E ASPASIA T Sa MM= KM S R
N N 80° 80° 80° Dennitsa D. 80° Y LO S Sz um U N éla yn H EG nya -U I BACHUE URO IA D d P ANAHIT CHKA PLANIT ors Klenova yr L CORONA M POMONA a D A ET CORONA o N Renpet IS r I R CORONA T sa T Mons EG FERONIA ET L I I H A . Thallo O A U u Tünde CORONA F S k L Mons 60° re R 60° 60° R a 60° . y j R E e u M Ivka a VACUNA GI l m O k . es E Allat Do O EARHART o i p e Margit N OTAU nt M T rsa CORONA a t a D E o I R Melia CORONA n o r o s M M .Wanda S H TA D a L O CORONA a n g I S Akn Mons o B . t Y a x r Mokosha N Rita e w U E M e A AUDRA D s R V s E S R l S VENUS FAKAHOTU a Mons L E E A l ES o GI K A T NIGHTINGALE I S N P O HM Cleopatra M RTUN A VÉNUSZ VENERA CORONA r V I L P FO PLANITIA ÂÅÍÅÐÀ s A o CORONA M e LA N P N n K a IT MOIRA s UM . a Hemera Dorsa A Iris DorsaBarsova 11 km a E IA TESSERA t t m A e a VENUŠE WENUS Hiei Chu n R a r A R E s T S DEMETER i A d ES D L A o Patera A r IS T N o R s r TA VIRIL CORONA s P s u e a L A N I T I A P p nt A o A L t e o N s BEIWE s M A ir u K A D G U Dan Baba-Jaga 1 : 45 000 000 E a CORONA D T N o M e or E ASPASIA t sa MM= KM S r . -

Providence: from Pronoia to Immanent Affirmation in John Calvin's Institutes of 1559
Providence: from pronoia to immanent affirmation in John Calvin's Institutes of 1559 The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Sanchez, Michelle Chaplin. 2014. Providence: from pronoia to immanent affirmation in John Calvin's Institutes of 1559. Doctoral dissertation, Harvard University. Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:12274125 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA Providence: from pronoia to immanent affirmation in John Calvin’s Institutes of 1559 A dissertation presented by Michelle Chaplin Sanchez to The Committee on the Study of Religion in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the subject of The Study of Religion Harvard University Cambridge, Massachusetts May 2014 © 2014 Michelle Chaplin Sanchez All rights reserved. Dissertation Advisor: Amy Hollywood Michelle Chaplin Sanchez Providence: from pronoia to immanent affirmation In John Calvin’s Institutes of 1559 Abstract Over the twentieth century and into the present, theorists of secularization and political theology have explored ways that theological arguments have shaped the social, ethical, economic, and political imaginaries of the modern West. In many of these studies, the doctrine of providence has come under scrutiny alongside related theological debates over of the nature of divine sovereignty, glory, the will, and the significance of immanent life in relation to divine transcendence. -
Capital Credits Member List
CAPITAL CREDITS MEMBER LIST RETIREMENT YEARS: 1984 to 1988 July 16, 2019 CUSTOMER CUSTOMER or NOTICE LOCATION REFUND NUMBER ORGANIZATION NAME(S) SENT DATE NUMBER ADDRESS CITY COUNTY YEARS 182313 C E POWERS & SONS 16969 UNAVAILABLE SCHENECTADY WASHINGTON 1986 100205 C Y O CAMP CHRISTINA 09/05/2014 4929 UNAVAILABLE SCHENECTADY BROWN 1984-1988 8796 UNAVAILABLE SCHENECTADY BROWN 1984-1988 8797 9491 E GRANDVIEW RD COLUMBUS BROWN 1984-1988 8798 UNAVAILABLE SCHENECTADY BROWN 1984-1988 181733 IVAN CABLE 09/08/2016 BETHEL CABLE 16904 635 CRABAPPLE CT NORTH VERNON JENNINGS 1984-1988 169030 ROBERT L. CABLE 09/08/2016 ORA I CABLE 11363 6485 W TODD CT NORTH VERNON JENNINGS 1984-1988 165644 ARLAND R. CAFFEE 09/08/2017 CHERRIE CAFFEE 11797 1117 E CUTSHALL RD SCOTTSBURG SCOTT 1984-1988 167564 MELVIN CAFFEE PEGGY CAFFEE 11259 UNAVAILABLE SCHENECTADY JACKSON 1985-1988 150900 MYRA J. CAGLE 09/07/2018 6647 3169 PLEASANT RUN RD HELTONVILLE LAWRENCE 1987-1988 181198 EDITH E. CAIN 16845 UNAVAILABLE SCHENECTADY LAWRENCE 1984-1985 104545 EDWIN CAIN 09/03/2015 NORMA C CAIN 15276 5260 N COUNTY ROAD 700 W SCIPIO JENNINGS 1984-1988 177471 GARY CAIN 16017 4787 N WINDDRIFT RD SCOTTSBURG SCOTT 1986 143815 GLENDA CAIN 09/07/2018 4488 2443 N COUNTY ROAD 1250 W NORMAN JACKSON 1987 108569 GLENDA L CAIN 01/01/2001 15104 7044 N COUNTY ROAD 550 E SEYMOUR JACKSON 1984-1985 169163 GUY CAIN 09/08/2016 PAMELA CAIN 12102 176 EASTERN HEIGHTS DR BEDFORD LAWRENCE 1984-1985 165634 J D CAIN 11794 UNAVAILABLE BEDFORD LAWRENCE 1984-1985 183504 JOHN F. -

Teaching the Reformations
Teaching the Reformations Edited by Christopher Metress Printed Edition of the Special Issue Published in Religions www.mdpi.com/journal/religions Teaching the Reformations Special Issue Editor Christopher Metress MDPI • Basel • Beijing • Wuhan • Barcelona • Belgrade Special Issue Editor Christopher Metress Samford University USA Editorial Office MDPI AG St. Alban-Anlage 66 Basel, Switzerland This edition is a reprint of the Special Issue published online in the open access journal Religions (ISSN 2077-1444) in 2017 (available at: http://www.mdpi.com/journal/religions/special_issues/reformations). For citation purposes, cite each article independently as indicated on the article page online and as indicated below: Author 1; Author 2. Article title. Journal Name. Year. Article number, page range. First Edition 2017 ISBN 978‐3‐03842‐522‐9 (Pbk) ISBN 978‐3‐03842‐523‐6 (PDF) Articles in this volume are Open Access and distributed under the Creative Commons Attribution license (CC BY), which allows users to download, copy and build upon published articles even for commercial purposes, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. The book taken as a whole is © 2018 MDPI, Basel, Switzerland, distributed under the terms and conditions of the Creative Commons license CC BY-NC-ND (http://creativecommons.org/licenses/by-nc-nd/4.0/). Table of Contents List of the Contributors ................................................................................................................................ -

6 Polygonal Impact Craters (Pics) 18 6.1 Definition of Polygonal Impact Craters
MASTERARBEIT Titel der Masterarbeit “Relative Age of Polygonal Impact Craters on Venus“ Verfasser Dipl.-Ing Gerhard Weihs BSc angestrebter akademischer Titel Master of Science (MSc) Wien, 2014 Studienkennzahl lt. Studienbuch: A 066 861 Studienrichtung lt. Studienblatt: Masterstudium Astronomie Betreuerin: Univ.-Prof. Dr. Maria G. Firneis Acknowledgement I gratefully acknowledge the thoughtful reviews by Univ.-Prof. Dr. Maria G. Firneis and Mag. Johannes J. Leitner, who significantly helped to improve the content and the style of this manuscript. Gerhard Weihs II Contents 1 Introduction 1 1.1 Aims of the Study . .1 1.2 Key Facts of Venus . .1 2 Venusian Surface 2 2.1 Geological Structures on Venusian Surface . .2 2.1.1 Main Elements of the Venusian Surface . .2 2.1.1.1 Volcanic Plains . .3 2.1.1.2 Intensely Deformed Terrains . .4 2.1.1.3 Coronae . .5 2.1.1.4 Impact Craters . .5 3 Geological History of the Venusian Surface 6 3.1 Short Overview of the Geological History . .6 3.2 Cratering Statistics . .7 3.3 Global Resurfacing of Venus . .8 3.3.1 Resurfacing Models . .8 3.3.2 Standard Model of Venusian Resurfacing . .9 4 Dating Planetary Surfaces 11 4.1 Methods of Dating Planetary Surfaces . 11 4.1.1 Absolute Dating - Chronology . 11 4.1.2 Relative Age Dating - Stratigraphy . 11 4.2 Chronology - Absolute Age Dating . 11 4.2.1 Using Radioactive Isotopes . 11 4.2.2 Using Crater Counting . 12 5 Impact Cratering Processes 15 5.1 The three Stages of Formation of Impact Craters . 15 5.1.1 Contact and Compression . -

Data Base of Impact Craters on Venus Based on Analysis of Magellan Radar Images and Altimetry Data
U.S. DEPARTMENT OF THE INTERIOR U.S. GEOLOGICAL SURVEY Data Base of Impact Craters on Venus Based On Analysis of Magellan Radar Images and Altimetry Data by Gerald G. Schaber - Emeritus Randolph L. Kirk Robert G. Strom Open-File Report 98-104 Revision of OFR 96-688 and of OFR 95-561 of the same title 1998 Prepared For the National Aeronautics and Space Administration Under NASA Contracts WO-8777 and W-18,727 This report is preliminary and has not been reviewed for conformity with U.S. Geological Survey editorial standards. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government___ Gerald G. Schaber, US Geological Survey - Emeritus, 2255 N. Gemini Drive, Flagstaff, AZ 86001 Randolph L Kirk, US Geological Survey, 2255 N. Gemini Drive, Flagstaff, AZ 86001 Robert G. Strom, Dept of Planetary Sciences, University ofArizona, Tucson AZ 85721 l|Table of ContentsPata Tables (NOTE; AN ELECTRONIC VERSION OF THIS OPEN FILE REPORT CAN BE SEEN & DOWNLOADED ON THE INTERNET AT: http: / /wwwf lag .wr .usgs. gov) Data Base of Impact Craters on Venus Table of Contents Title Page Introduction The Crater Database Categories Included in the Database Names Modification State Crater Type Elevation Download the Database General Information References Cited Venus Crater Database Introduction The NASA Magellan spacecraft provided synthetic aperture radar (SAR) image coverage of 98% of the surface of the planet Venus, in addition to topography and several types of physical property data on the venusian surface (e.g., radar reflectivity, radar backscatter, emissivity, and rms slopes).(See Special Magellan Issue of J. -
Cross-Spectral Synergy and Consonant Identification (A)
View metadata,Downloaded citation and from similar orbit.dtu.dk papers on:at core.ac.uk Dec 17, 2017 brought to you by CORE provided by Online Research Database In Technology Cross-spectral synergy and consonant identification (A) Christiansen, Thomas Ulrich; Greenberg, Steven Published in: Acoustical Society of America. Journal Link to article, DOI: 10.1121/1.2935680 Publication date: 2008 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Christiansen, T. U., & Greenberg, S. (2008). Cross-spectral synergy and consonant identification (A). Acoustical Society of America. Journal, 123(5), 3850-3850. DOI: 10.1121/1.2935680 General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. FRIDAY MORNING, 4 JULY 2008 ROOM 242B, 8:00 A.M. TO 3:00 P.M. Session 5aAAa Architectural Acoustics: New Frontiers in Room Acoustical Modeling I Murray Hodgson, Cochair The University of British Columbia, Department of Electrical and Computer Engineering, 2332 Main Mall, Vancouver, BC V6T 1Z4, Canada Vincent Valeau, Cochair Laboratoire d’Etudes Aérodynamiques (LEA), Université de Poitiers - ENSMA - CNRS, Bâtiment K, 40 Avenue du Recteur Pineau, Poitiers, F-86022, France Contributed Papers 8:00 that multiresolution geometry provides more spatially accurate results than 5aAAa1.