Araneae, Mimetidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Checklist of the Non -Acarine Arachnids
Original Research A CHECKLIST OF THE NON -A C A RINE A R A CHNIDS (CHELICER A T A : AR A CHNID A ) OF THE DE HOOP NA TURE RESERVE , WESTERN CA PE PROVINCE , SOUTH AFRIC A Authors: ABSTRACT Charles R. Haddad1 As part of the South African National Survey of Arachnida (SANSA) in conserved areas, arachnids Ansie S. Dippenaar- were collected in the De Hoop Nature Reserve in the Western Cape Province, South Africa. The Schoeman2 survey was carried out between 1999 and 2007, and consisted of five intensive surveys between Affiliations: two and 12 days in duration. Arachnids were sampled in five broad habitat types, namely fynbos, 1Department of Zoology & wetlands, i.e. De Hoop Vlei, Eucalyptus plantations at Potberg and Cupido’s Kraal, coastal dunes Entomology University of near Koppie Alleen and the intertidal zone at Koppie Alleen. A total of 274 species representing the Free State, five orders, 65 families and 191 determined genera were collected, of which spiders (Araneae) South Africa were the dominant taxon (252 spp., 174 genera, 53 families). The most species rich families collected were the Salticidae (32 spp.), Thomisidae (26 spp.), Gnaphosidae (21 spp.), Araneidae (18 2 Biosystematics: spp.), Theridiidae (16 spp.) and Corinnidae (15 spp.). Notes are provided on the most commonly Arachnology collected arachnids in each habitat. ARC - Plant Protection Research Institute Conservation implications: This study provides valuable baseline data on arachnids conserved South Africa in De Hoop Nature Reserve, which can be used for future assessments of habitat transformation, 2Department of Zoology & alien invasive species and climate change on arachnid biodiversity. -

Casting a Sticky Trap: SPIDERS and Their Predatory Ways
Casting a Sticky Trap: SPIDERS and Their Predatory Ways Bennett C. Moulder, ISM Research Associate and Professor Emeritus, Illinois College, Jacksonville, Illinois ating insects and other small sticky silk, capable of trapping and holding to the spot, and impales the insect with its arthropods is what spiders do for even relatively large an powerful insect prey. long chelicerae. First using her mouthparts a living. That spiders are an Damage to the orb caused by wind or strug- to cut a small slit in the tube, the spider enormously successful group of gling prey can be quickly repaired, or the then pulls the insect inside. After her meal, animals is testimony to how efficient they entire can be web taken down and replaced. the spider repairs the tear in the tube, re- Eare at catching their prey. Most insects move Many orb weavers replace their tattered web sumes her position below ground, and swiftly and, for their size, are quite powerful. with a new one every day. awaits the next victim. “For spiders to capture such prey, they have, Other spiders do not utilize webs for prey Mastophora, the bolas spider, is a mem- as a group, developed an impressive arsenal capture. Crab spiders, perfectly camouflaged ber of the family Araneidae, the typical orb of weapons and a variety of strategies. against their background, lie in wait on flow- weavers. This small spider has abandoned Except for one small family (Ul- ers or on the bark of trees to ambush unsus- the practice of orb web construction alto- oboridae), all spiders in Illinois have venom pecting insects. -

A Protocol for Online Documentation of Spider Biodiversity Inventories Applied to a Mexican Tropical Wet Forest (Araneae, Araneomorphae)
Zootaxa 4722 (3): 241–269 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2020 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4722.3.2 http://zoobank.org/urn:lsid:zoobank.org:pub:6AC6E70B-6E6A-4D46-9C8A-2260B929E471 A protocol for online documentation of spider biodiversity inventories applied to a Mexican tropical wet forest (Araneae, Araneomorphae) FERNANDO ÁLVAREZ-PADILLA1, 2, M. ANTONIO GALÁN-SÁNCHEZ1 & F. JAVIER SALGUEIRO- SEPÚLVEDA1 1Laboratorio de Aracnología, Facultad de Ciencias, Departamento de Biología Comparada, Universidad Nacional Autónoma de México, Circuito Exterior s/n, Colonia Copilco el Bajo. C. P. 04510. Del. Coyoacán, Ciudad de México, México. E-mail: [email protected] 2Corresponding author Abstract Spider community inventories have relatively well-established standardized collecting protocols. Such protocols set rules for the orderly acquisition of samples to estimate community parameters and to establish comparisons between areas. These methods have been tested worldwide, providing useful data for inventory planning and optimal sampling allocation efforts. The taxonomic counterpart of biodiversity inventories has received considerably less attention. Species lists and their relative abundances are the only link between the community parameters resulting from a biotic inventory and the biology of the species that live there. However, this connection is lost or speculative at best for species only partially identified (e. g., to genus but not to species). This link is particularly important for diverse tropical regions were many taxa are undescribed or little known such as spiders. One approach to this problem has been the development of biodiversity inventory websites that document the morphology of the species with digital images organized as standard views. -
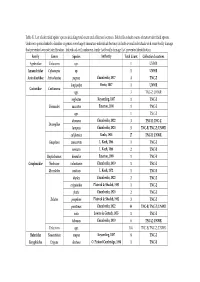
Table S1. List of Identified Spider Species Including Total Count and Collection Locations. Bold Cells Include Counts of Mature Identified Species
Table S1. List of identified spider species including total count and collection locations. Bold cells include counts of mature identified species. Unknown species linked to families or genera were largely immature individuals but may include several individuals with some bodily damage that prevented accurate identification. Individuals with unknown family had bodily damage that prevented identification. Family Genus Species Authority Total Count Collection Locations Agelenidae Unknown spp. 1 UNWR Amaurobiidae Cybaeopsis sp. 1 UNWR Antrodiaetidae Antrodiaetus pugnax Chamberlin, 1917 4 TNC-Z longipalpa Hentz, 1847 1 UNWR Corinnidae Castianeira spp. 3 TNC-Z; UNWR neglectus Keyserling, 1887 1 TNC-Z Drassodes saccatus Emerton, 1890 1 TNC-Z spp. 1 TNC-Z dromeus Chamberlin, 1922 3 TNC-B; TNC-Z Drassyllus lamprus Chamberlin, 1920 3 TNC-B; TNC-Z; UNWR californica Banks, 1904 17 TNC-B; UNWR Gnaphosa muscorum L. Koch, 1866 1 TNC-Z sericata L. Koch, 1866 2 TNC-B Haplodrassus hiemalis Emerton, 1909 1 TNC-B Gnaphosidae Nodocion voluntaries Chamberlin, 1919 1 TNC-Z Urozelotes rusticus L. Koch, 1872 1 TNC-B duplex Chamberlin, 1922 2 TNC-Z exiguioides Platnick & Shadab, 1983 1 TNC-Z fratis Chamberlin, 1920 2 TNC-Z Zelotes josephine Platnick & Shadab, 1983 3 TNC-Z puritanus Chamberlin, 1922 44 TNC-B; TNC-Z; UNWR sula Lowrie & Gertsch, 1955 1 TNC-Z tubuous Chamberlin, 1919 6 TNC-Z; UNWR Unknown spp. 184 TNC-B; TNC-Z; UNWR Hahniidae Neoantistea magna Keyserling, 1887 8 TNC-Z Linyphiidae Erigone dentosa O. Pickard-Cambridge, 1894 1 TNC-B spp. 1 TNC-Z Unknown spp. 13 TNC-Z; UNWR mccooki Montgomery, 1904 95 TNC-B; TNC-Z; UNWR Schizocosa minnesotensis Gertsch, 1934 25 TNC-B Lycosidae spp. -

Common Kansas Spiders
A Pocket Guide to Common Kansas Spiders By Hank Guarisco Photos by Hank Guarisco Funded by Westar Energy Green Team, American Arachnological Society and the Chickadee Checkoff Published by the Friends of the Great Plains Nature Center i Table of Contents Introduction • 2 Arachnophobia • 3 Spider Anatomy • 4 House Spiders • 5 Hunting Spiders • 5 Venomous Spiders • 6-7 Spider Webs • 8-9 Other Arachnids • 9-12 Species accounts • 13 Texas Brown Tarantula • 14 Brown Recluse • 15 Northern Black Widow • 16 Southern & Western Black Widows • 17-18 Woodlouse Spider • 19 Truncated Cellar Spider • 20 Elongated Cellar Spider • 21 Common Cellar Spider • 22 Checkered Cobweb Weaver • 23 Quasi-social Cobweb Spider • 24 Carolina Wolf Spider • 25 Striped Wolf Spider • 26 Dotted Wolf Spider • 27 Western Lance Spider • 28 Common Nurseryweb Spider • 29 Tufted Nurseryweb Spider • 30 Giant Fishing Spider • 31 Six-spotted Fishing Spider • 32 Garden Ghost Spider Cover Photo: Cherokee Star-bellied Orbweaver ii Eastern Funnelweb Spider • 33 Eastern and Western Parson Spiders • 34 Garden Ghost Spider • 35 Bark Crab Spider • 36 Prairie Crab Spider • 37 Texas Crab Spider • 38 Black-banded Crab Spider • 39 Ridge-faced Flower Spider • 40 Striped Lynx Spider • 41 Black-banded Common and Convict Zebra Spiders • 42 Crab Spider Dimorphic Jumping Spider • 43 Bold Jumping Spider • 44 Apache Jumping Spider • 45 Prairie Jumping Spider • 46 Emerald Jumping Spider • 47 Bark Jumping Spider • 48 Puritan Pirate Spider • 49 Eastern and Four-lined Pirate Spiders • 50 Orchard Spider • 51 Castleback Orbweaver • 52 Triangulate Orbweaver • 53 Common & Cherokee Star-bellied Orbweavers • 54 Black & Yellow Garden Spider • 55 Banded Garden Spider • 56 Marbled Orbweaver • 57 Eastern Arboreal Orbweaver • 58 Western Arboreal Orbweaver • 59 Furrow Orbweaver • 60 Eastern Labyrinth Orbweaver • 61 Giant Long-jawed Orbweaver • 62 Silver Long-jawed Orbweaver • 63 Bowl and Doily Spider • 64 Filmy Dome Spider • 66 References • 67 Pocket Guides • 68-69 1 Introduction This is a guide to the most common spiders found in Kansas. -

Spider Community Composition and Structure in a Shrub-Steppe Ecosystem: the Effects of Prey Availability and Shrub Architecture
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 5-2012 Spider Community Composition and Structure In A Shrub-Steppe Ecosystem: The Effects of Prey Availability and Shrub Architecture Lori R. Spears Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Philosophy Commons Recommended Citation Spears, Lori R., "Spider Community Composition and Structure In A Shrub-Steppe Ecosystem: The Effects of Prey Availability and Shrub Architecture" (2012). All Graduate Theses and Dissertations. 1207. https://digitalcommons.usu.edu/etd/1207 This Dissertation is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. SPIDER COMMUNITY COMPOSITION AND STRUCTURE IN A SHRUB-STEPPE ECOSYSTEM: THE EFFECTS OF PREY AVAILABILITY AND SHRUB ARCHITECTURE by Lori R. Spears A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in Ecology Approved: ___________________________ ___________________________ James A. MacMahon Edward W. Evans Major Professor Committee Member ___________________________ ___________________________ S.K. Morgan Ernest Ethan P. White Committee Member Committee Member ___________________________ ___________________________ Eugene W. Schupp Mark R. McLellan Committee Member Vice President for Research and Dean of the School of Graduate Studies UTAH STATE UNIVERSITY Logan, Utah 2012 ii Copyright © Lori R. Spears 2012 All Rights Reserved iii ABSTRACT Spider Community Composition and Structure in a Shrub-Steppe Ecosystem: The Effects of Prey Availability and Shrub Architecture by Lori R. -

Onetouch 4.0 Sanned Documents
Anna. Rev. Ecol. Swf. 1991. 22:565-92 SYSTEMATICS AND EVOLUTION OF SPIDERS (ARANEAE)* • Jonathan A. Coddington Department of Entomology, National Museum of Natural History. Smithsonian Institution, Washington, DC 20560 Herbert W. Levi Museum of Comparative Zoology, Harvard University, Cambridge, Massachusetts 02138 KEY WORDS: taxonomy, phytogeny, cladistics, biology, diversity INTRODUCTION In the last 15 years understanding of the higher systematics of Araneae has changed greatly. Large classical superfamilies and families have turned out to be poly- or paraphyletic; posited relationships were often based on sym- plesiomorphies. In this brief review we summarize current taxonomic and phylogenetic knowledge and suggest where future efforts might profitably be concentrated. We lack space to discuss fully all the clades mentioned, and the cited numbers of described taxa are only approximate. Other aspects of spider biology have been summarized by Barth (7), Eberhard (47), Jackson & Parks (72), Nentwig (105), Nyffeler & Benz (106), Riechert & Lockley (134), Shear (149) and Turnbull (160). Diversity, Paleontology, Descriptive Work, Importance The order Araneae ranks seventh in global diversity after the five largest insect orders (Coleoptera, Hymenoptera, Lepidoptera, Diptera, Hemiptera) and Acari among the arachnids (111) in terms of species described or an- *The US government has the right to retain a nonexclusive, royalty free license in and to any copyright covering this paper. 565 566 CODDINGTON & LEVI ticipated. Spiders are among the most diverse groups on earth. Among these taxa, spiders are exceptional for their complete dependence on predation as a trophic strategy. In contrast, the diversity of insects and mites may result from their diversity in dietary strategies•notably phytophagy and parasitism (104). -

Riparian Spider Communities As Indicators of Stream Ecosystem Condition in the Río Piedras Watershed of Puerto Rico
Actual Biol Volumen 39 / Numero 107, 2017 Artículo científi co completo Riparian spider communities as indicators of stream ecosystem condition in the Río Piedras watershed of Puerto Rico Comunidades de arañas ribereñas como indicadores de la condición de los ecosistemas fluviales en la cuenca del Río Piedras de Puerto Rico Roberto Reyes-Maldonado1,3, José A. Sánchez-Ruiz2,4, Alonso Ramírez2,5 Sean P. Kelly*1,6 Abstract Human degradation of stream ecosystems has led to the creation of a number of methods to assess the severity of such anthropogenic impacts. Biomonitoring protocols that utilize aquatic organisms, in particular macroinvertebrates, are used worldwide as a way to evaluate stream ecosystems. Despite the various benefits these methods provide, they only take into account the stream channel, ignoring altogether the condition of the riparian zone. Other methods look at physical characteristics of both the riparian area and the stream, but ignore biota. Riparian consumers such as spiders have been proposed as potential bioindicators because they could provide a more holistic alternative for assessing stream impair- ment. Our aim was to determine whether changes in riparian spider communities could be used as indicators to separate sites with different levels of impact along an urban gradient. We conducted correlation analyses of riparian spider commu- nity metrics (abundance and species richness) and the percent of vegetation cover in subwatersheds with varying levels of urbanization, along with three other popular stream monitoring protocols. We found a clear difference in spider com- munity composition among subwatersheds, with an overall trend for lower richness and abundances in more impacted sites. -

Variation in Arthropod Communities in Response to Urbanization: Seven Years of Arthropod Monitoring in a Desert City
Variation in Arthropod Communities in Response to Urbanization: Seven Years of Arthropod Monitoring in a Desert City By: Christofer Bang, Stanley H. Faeth Bang, C. and Faeth, S.H. 2011. Variation in arthropod communities in response to urbanization: Seven years of arthropod monitoring in a desert city. Landscape and Urban Planning 103(3-4): 383-399. Made available courtesy of Elsevier: http://dx.doi.org/10.1016/j.landurbplan.2011.08.013 ***© Elsevier. Reprinted with permission. No further reproduction is authorized without written permission from Elsevier. This version of the document is not the version of record. Figures and/or pictures may be missing from this format of the document. *** This is the author’s version of a work that was accepted for publication in Landscape and Urban Planning. Changes resulting from the publishing process, such as peer review, editing, corrections, structural formatting, and other quality control mechanisms may not be reflected in this document. Changes may have been made to this work since it was submitted for publication. A definitive version was subsequently published in Landscape and Urban Planning, Volume 103, Issue 3-4, (2011) DOI: 10.1016/j.landurbplan.2011.08.013 Abstract: Continuous monitoring is essential to understand dynamics of biological communities in response to urbanization, and to provide guidance in landscape planning for conserving urban biodiversity. Arthropods serve this purpose because they are abundant and diverse in urban areas, and relatively easy to collect. Over seven years, in the Central Arizona Phoenix area, arthropod communities in three urban habitat categories were collected and compared to arthropods in natural desert using pitfall traps and non-parametric analyses. -

Anthropod Community Associated with the Webs of the Subsocial Spider Anelosimus Studiosus
Georgia Southern University Digital Commons@Georgia Southern Electronic Theses and Dissertations Graduate Studies, Jack N. Averitt College of Fall 2008 Anthropod Community Associated with the Webs of the Subsocial Spider Anelosimus Studiosus Sarah Natalie Mock Follow this and additional works at: https://digitalcommons.georgiasouthern.edu/etd Recommended Citation Mock, Sarah Natalie, "Anthropod Community Associated with the Webs of the Subsocial Spider Anelosimus Studiosus" (2008). Electronic Theses and Dissertations. 702. https://digitalcommons.georgiasouthern.edu/etd/702 This thesis (open access) is brought to you for free and open access by the Graduate Studies, Jack N. Averitt College of at Digital Commons@Georgia Southern. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons@Georgia Southern. For more information, please contact [email protected]. THE ARTHROPOD COMMUNITY ASSOCIATED WITH THE WEBS OF THE SUBSOCIAL SPIDER ANELOSIMUS STUDIOSUS by SARAH N. MOCK (Under the Direction of Alan Harvey) ABSTRACT Anelosimus studiosus (Theridiidae) is a subsocial spider that has a diverse arthropod fauna associated with its webs. From south Georgia, I identified 1006 arthropods representing 105 species living with A. studiosus , and 40 species that were prey items from 250 webs. The arthropods seen in A. studiosus webs represented a distinct community from the arthropods on the tree. I found that Barronopsis barrowsi (Agelenidae) and Frontinella pyramitela was similar to A. studiosus in web structure and that B. barrowsi webs contained multiple arthropods. Also, previously known as asocial, B. barrowsi demonstrated sociality in having multiple adults per web. Lastly, the inquiline communities in the webs of A.studiosus and B.barronopsis contained many different feeding guilds, including herbivores, omnivores, generalist predators, kleptoparasites, and aranievores. -

A Checklist of Maine Spiders (Arachnida: Araneae)
A CHECKLIST OF MAINE SPIDERS (ARACHNIDA: ARANEAE) By Daniel T. Jennings Charlene P. Donahue Forest Health and Monitoring Maine Forest Service Technical Report No. 47 MAINE DEPARTMENT OF AGRICULTURE, CONSERVATION AND FORESTRY September 2020 Augusta, Maine Online version of this report available from: https://www.maine.gov/dacf/mfs/publications/fhm_pubs.htm Requests for copies should be made to: Maine Forest Service Division of Forest Health & Monitoring 168 State House Station Augusta, Maine 04333-0168 Phone: (207) 287-2431 Printed under appropriation number: 013-01A-2FHM-52 Issued 09/2020 Initial printing of 25 This product was made possible in part by funding from the U.S. Department of Agriculture. Forest health programs in the Maine Forest Service, Department of Agriculture Conservation and Forestry are supported and conducted in partnership with the USDA, the University of Maine, cooperating landowners, resource managers, and citizen volunteers. This institution is prohibited from discrimination based on race, color, national origin, sex, age, or disability. 2 A CHECKLIST OF MAINE SPIDERS (ARACHNIDA: ARANEAE) 1 2 DANIEL T. JENNINGS and CHARLENE P. DONAHUE ____________________________________ 1 Daniel T. Jennings, retired, USDA, Forest Service, Northern Forest Experiment Station. Passed away September 14, 2020 2 Charlene P. Donahue, retired, Department of Agriculture, Conservation and Forestry – Maine Forest Service. Corresponding Author [email protected] 4 Table of Contents Abstract 1 Introduction 1 Figure 1. Map of State of Maine -

Fossils – Adriano Kury’S Harvestman Overviews and the Third Edition of the Manual of Acarology for Mites
1 A summary list of fossil spiders and their relatives compiled by Jason A. Dunlop (Berlin), David Penney (Manchester) & Denise Jekel (Berlin) with additional contributions from Lyall I. Anderson, Simon J. Braddy, James C. Lamsdell, Paul A. Selden & O. Erik Tetlie Suggested citation: Dunlop, J. A., Penney, D. & Jekel, D. 2012. A summary list of fossil spiders and their relatives. In Platnick, N. I. (ed.) The world spider catalog, version 13.0 American Museum of Natural History, online at http://research.amnh.org/entomology/spiders/catalog/index.html Last updated: 20.06.2012 INTRODUCTION Fossil spiders have not been fully cataloged since Bonnet’s Bibliographia Araneorum and are not included in the current Catalog. Since Bonnet’s time there has been considerable progress in our understanding of the fossil record of spiders – and other arachnids – and numerous new taxa have been described. For an overview see Dunlop & Penney (2012). Spiders remain the single largest fossil group, but our aim here is to offer a summary list of all fossil Chelicerata in their current systematic position; as a first step towards the eventual goal of combining fossil and Recent data within a single arachnological resource. To integrate our data as smoothly as possible with standards used for living spiders, our list for Araneae follows the names and sequence of families adopted in the Platnick Catalog. For this reason some of the family groups proposed in Wunderlich’s (2004, 2008) monographs of amber and copal spiders are not reflected here, and we encourage the reader to consult these studies for details and alternative opinions.