The Evolution of Corneal and Refractive Surgery with the Femtosecond Laser
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Intraocular Pressure During Phacoemulsification
J CATARACT REFRACT SURG - VOL 32, FEBRUARY 2006 Intraocular pressure during phacoemulsification Christopher Khng, MD, Mark Packer, MD, I. Howard Fine, MD, Richard S. Hoffman, MD, Fernando B. Moreira, MD PURPOSE: To assess changes in intraocular pressure (IOP) during standard coaxial or bimanual micro- incision phacoemulsification. SETTING: Oregon Eye Center, Eugene, Oregon, USA. METHODS: Bimanual microincision phacoemulsification (microphaco) was performed in 3 cadaver eyes, and standard coaxial phacoemulsification was performed in 1 cadaver eye. A pressure transducer placed in the vitreous cavity recorded IOP at 100 readings per second. The phacoemulsification pro- cedure was broken down into 8 stages, and mean IOP was calculated across each stage. Intraocular pressure was measured during bimanual microphaco through 2 different incision sizes and with and without the Cruise Control (Staar Surgical) connected to the aspiration line. RESULTS: Intraocular pressure exceeded 60 mm Hg (retinal perfusion pressure) during both standard coaxial and bimanual microphaco procedures. The highest IOP occurred during hydrodissection, oph- thalmic viscosurgical device injection, and intraocular lens insertion. For the 8 stages of the phaco- emulsification procedure delineated in this study, IOP was lower for at least 1 of the bimanual microphaco eyes compared with the standard coaxial phaco eye in 4 of the stages (hydro steps, nu- clear disassembly, irritation/aspiration, anterior chamber reformation). CONCLUSION: There was no consistent difference in IOP between the bimanual microphaco eyes and the eye that had standard coaxial phacoemulsification. Bimanual microincision phacoemul- sification appears to be as safe as standard small incision phacoemulsification with regard to IOP. J Cataract Refract Surg 2006; 32:301–308 Q 2006 ASCRS and ESCRS Bimanual microincision phacoemulsification, defined as capable of insertion through these microincisions become cataract extraction through 2 incisions of less than 1.5 mm more widely available. -

Eyes Before Cataract Surgery
HIGH-RISK EYES Recognising ‘high-risk’ eyes before cataract surgery Parikshit Gogate Mark Wood Head, Department of Paediatric Ophthalmology, Community Consultant Ophthalmologist, CCBRT Hospital, Eye Care, HV Desai Eye Hospital, Pune 411028, India. Box 23310, Dar es Salaam, Tanzania. Email: [email protected] Email: [email protected] Certain eyes are at a higher risk of compli- Conjunctivitis should be treated with cation during cataract surgery. Operations topical antibiotics prior to intraocular on such ‘high-risk’ eyes are also more likely surgery. to yield a poor visual outcome (defined as Noble Bruce best corrected vision less than 6/60 after Potential visualisation surgery).1 Learning to recognise when eyes are at problems during surgery greater risk, and acting accordingly, will help Corneal opacity you to avoid complications. Even so, before Leucoma-grade opacity will make your task the operation takes place, it is good practice Conjunctivitis extremely difficult. You will find it difficult to to explain to such patients that a poor see details, in particular the capsulotomy. outcome is a possibility. This makes these There may be residual lens matter • Measuring intraocular pressure. It is patients’ expectations more realistic and remaining in the bag, which will be difficult important to measure intraocular pressure improves postoperative compliance and to see. It will also be challenging to place in all patients, for example to identify follow-up. In most cases, patients who are the intraocular lens (IOL) in the posterior glaucoma. blind with complicated cataract will be chamber with both haptics under the iris. • A fundus examination. The fundus can happy with even a modest improvement of be seen through all but the densest Patients suffering from trachoma with their vision. -

A Study on the Efficacy and Corneal Penetration of Besifloxacin in Routine Cataract Surgery Cases
A STUDY ON THE EFFICACY AND CORNEAL PENETRATION OF BESIFLOXACIN IN ROUTINE CATARACT SURGERY CASES Thesis submitted to THE TAMILNADU Dr.M.G.R. MEDICAL UNIVERSITY Chennai - 600 032. In partial fulfillment of the requirements For the award of the degree of MASTER OF PHARMACY in PHARMACY PRACTICE Submitted by Reg. No.: 261640151 DEPARTMENT OF PHARMACY PRACTICE PERIYAR COLLEGE OF PHARMACEUTICAL SCIENCES TIRUCHIRAPPALLI – 620 021. An ISO 9001:2015 Certified Institution SEPTEMBER – 2018 Dr. A. M. ISMAIL, M.Pharm., Ph.D., Professor Emeritus Periyar College of Pharmaceutical Sciences Tiruchirappalli – 620 021. CERTIFICATE This is to certify that the thesis entitled “A STUDY ON THE EFFICACY AND CORNEAL PENETRATION OF BESIFLOXACIN IN ROUTINE CATARACT SURGERY CASES” submitted by B. NIVETHA, B. Pharm., during September 2018 for the award of the degree of “MASTER OF PHARMACY in PHARMACY PRACTICE” under the Tamilnadu Dr.M.G.R. Medical University, Chennai is a bonafide record of research work done in the Department of Pharmacy Practice, Periyar College of Pharmaceutical Sciences and at Vasan Eye Care Hospital, Tiruchirappalli under my guidance and direct supervision during the academic year 2017-18. Place: Tiruchirappalli – 21. Date: 10th Sep 2018 (Dr. A. M. ISMAIL) Dr. R. SENTHAMARAI, M.Pharm., Ph.D., Principal Periyar College of Pharmaceutical sciences Tiruchirappalli – 620 021. CERTIFICATE This is to certify that the thesis entitled “A STUDY ON THE EFFICACY AND CORNEAL PENETRATION OF BESIFLOXACIN IN ROUTINE CATARACT SURGERY CASES” submitted by B. NIVETHA, B. Pharm., during September 2018 for the award of the degree of “MASTER OF PHARMACY in PHARMACY PRACTICE” under the Tamilnadu Dr.M.G.R. -

Single-Use Ophthalmic Surgical Products Ophthalmic
ophthalmic Single-use. Products to meet the demands of modern ophthalmic surgery techniques. single-use ophthalmic surgical products ophthalmic Delivering innovation in ophthalmic surgery Sterimedix is a manufacturer of single use surgical cannula The requirements of eye surgery place a great responsibility products based in the UK. With over 25 year’s experience on the manufacturer to maintain the highest standards of of manufacturing single-use devices, primarily for quality. The regulatory environment in which the Company ophthalmology, the Company has a rare depth of expertise operates demands the best manufacturing and control in new product development. standards. QUALITY ASSURANCE. Sterimedix Limited is committed to producing products of the highest quality. The company’s quality systems and practices are regularly audited and have ISO 13485, ISO 9001 and CE accreditations together with United States FDA registration, and the appropriate approvals in all other markets where Sterimedix is active. 2 Continuous developments in response to our customers’ requirements; continued growth from excellent product quality and customer service. Sterimedix. Dedicated to supporting our distributors and their customers Our quest is to continually improve both our service and Sterimedix products are packed sterile in a single blister products, supplying innovative single use ophthalmic in space saving boxes designed to minimise waste. instruments at competitive prices, presented in user friendly Our products may also be supplied non-sterile in unique packaging, and meeting our customers’ delivery formats compatible with the requirements of kit packers. requirements. This catalogue contains details of the entire Sterimedix range, including unique new products designed to work with the latest techniques, in addition to a tried and trusted portfolio of single use products for all ophthalmic procedures. -

Techniques Management of Posterior Polar Cataract
techniques Management of posterior polar cataract I. Howard Fine, MD, Mark Packer, MD, Richard S. Hoffman, MD In this technique for managing posterior polar cataract, extreme care is taken not to overpressurize the anterior chamber or capsular bag to prevent posterior cap- sule rupture. Minimal hydrodissection and hydrodelineation are performed. The nucleus is extracted using minimal ultrasound energy. Viscodissection is used as a primary technique to mobilize the epinucleus and cortex. A protective layer is preserved over the posterior polar region until the conclusion of the extraction procedure to minimize the risk of loss of lens material into the vitreous cavity in the case of a capsule defect. J Cataract Refract Surg 2003; 29:16–19 © 2003 ASCRS and ESCRS he posterior polar cataract is one of the most diffi- epinucleus and cortex to avoid unnecessary pressure on Tcult challenges for cataract surgeons because of the the posterior capsule and protect the region of greatest high likelihood of posterior capsule rupture. Osher and potential weakness throughout the procedure. Viscodis- coauthors1 report a 26% incidence of capsule rupture in section of epinuclear and cortical material involves peel- a series of 31 cases and Vasavada and Singh,2 a 36% ing away layers with a cushion of dispersive viscoelastic incidence in a series of 22 cases. material that partitions the lens capsule from the activity Both stationary and progressive posterior polar cat- inside. aracts may become symptomatic. Frequently, this con- dition does not become a problem for patients until they are entering young adulthood and become troubled by Surgical Technique glare and other disturbing visual images, especially when Incision and Capsulorhexis driving at night. -
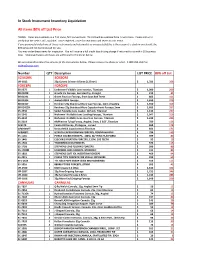
In Stock Instrument Inventory Liquidation All Items 80% Off List Price
In Stock Instrument Inventory Liquidation All items 80% off List Price TERMS: Items are available on a first come, first served basis. This list will be updated from time to time. Please call us to verify that the item is still available. Once depleted, price for new items will revert to LIST PRICE. If you previously trialed one of these instruments and returned it as unacceptable(this is the reason it is stuck in our stock), the 80% price will not be honoured for you. You may order these items for inspection. You will receive a full credit less shiping charge if returned to us within 10 business days. Shipping charges and taxes are additional to the prices below. We are pleased to email/fax pictures of the instruments below. Please request by phone or email. 1-800-263-3557 or, [email protected] Number QTY Description LIST PRICE 80% off List SCISSORS SCISSORS VR-1812 25g Curved Scissors 0.5mm (1.25mm) $ 1,781 356 FORCEPS FORCEPS 05-2375 1 Lindstrom Foldable Lens Inserter, Titanium $ 1,000 200 08-01208 1 Graefe Iris Forceps, Serrated Tip, Straight $ 212 43 08-01291 1 Green Fixation Forceps, 5mm Jaws 8x9 Teeth $ 803 161 08-01304 1 Ambati DSEK Forceps $ 1,350 270 08-01417 1 Kershner 23g Stainless Micro Cap Forceps, 1mm irrigating $ 1,550 310 08-01422N 1 Kershner 23g Stainless Micro Capsulorrhexis Forceps,1mm $ 1,685 337 05-2336 2 Seibel Foldable Lens Loader, W/Lock, Titanium $ 1,132 227 05-2342 1 Nichamin I Foldable Lens Loading Forceps, Titanium $ 1,047 210 05-2344 2 Nichamin I Foldable Lens Insertion Forceps, Titanium $ 1,229 246 05-2119 -

Analysis of Factors Associated with Vision After Cataract Surgery in Chronic Renal Failure Patients on Dialysis
Yin et al. BMC Ophthalmology (2020) 20:211 https://doi.org/10.1186/s12886-020-01479-w RESEARCH ARTICLE Open Access Analysis of factors associated with vision after cataract surgery in chronic renal failure patients on dialysis Songtao Yin, Jie Zhang, Xia Hua, Guannan Huang, Biyun Jia, Yang Liu, Yao Ma and Long Su* Abstract Background: To analyze the related factors of visual acuity after phacoemulsification and intraocular lens implantation in chronic renal failure (CRF) patients. Methods: We retrospectively analyzed 42 patients (51 eyes) with CRF (failure, uremia) on hemodialysis or peritoneal dialysis and 40 patients (50 eyes) without CRF as a control group. Each individual underwent physical and laboratory examinations including best corrected visual acuity (BCVA), slit lamp examination, intraocular pressure, corneal endothelial cell count, fundus examination and optical coherence tomography (OCT) for macular examination. The patients with abnormal platelet, liver and kidney function, coagulation function received treatment accordingly to reduce the perioperative risk. All patients underwent phacoemulsification with IOL implantation. Follow-up examinations were performed at 1 week, 1 month and 3 months after surgery and included BCVA, slit lamp examination, noncontact IOP, dilated fundus examination and OCT of the macula. Results: In control group the preoperative RBC, HB, Cr, and urea values were not associated with the pre- or postoperative BCVA. The RBC, HB, Cr, urea, SBP, DBP, preoperative BCVA and postoperative BCVA values were all significantly different between CRF and control group(P <0.05). Conclusion: In CRF patients, the RBC, HB, Cr and Urea indexes should be monitored before the cataract operation for guarded visual outcome. -

Case-Discussions-In
discussions Highlights from a CME Symposium held during the American Academy of Ophthalmology 2010 Meeting Program Chairman and Moderator Dale K. Heuer, MD Faculty Donald L. Budenz, MD, MPH Eric D. Donnenfeld, MD Richard Lewis, MD Sponsored by The New York Eye and Ear Infirmary Original Release: March 1, 2011 Last Review: February 16, 2011 Expiration: March 31, 2012 In joint sponsorship with MedEdicus LLC Part 2 of 2 This continuing medical education activity is supported through an unrestricted educational grant from Pfizer Inc March 2011 case discussions faculty Eric D. Donnenfeld, MD, FAAO program chairman Founding Partner Dale K. Heuer, MD and moderator Ophthalmic Consultants of Long Island Professor and Chairman of Ophthalmology Rockville Centre, New York Medical College of Wisconsin Clinical Professor of Ophthalmology Director NYU Langone Medical Center Froedtert Hospital and the Medical College New York, New York of Wisconsin Eye Institute Trustee Milwaukee, Wisconsin Dartmouth Medical School Hanover, New Hampshire Donald L. Budenz, MD, MPH Professor of Ophthalmology, Epidemiology, Richard Lewis, MD and Public Health Co-Founder and Director University of Miami Miller School of Medicine Capital City Surgery Center Highlights from a CME Symposium Associate Medical Director Sacramento, California Bascom Palmer Eye Institute Past President held during the American Academy of Miami, Florida American Glaucoma Society Ophthalmology 2010 Meeting learning method and medium qualifications or suitability. The intention is to provide full disclosure of any potential conflict This educational activity consists of a supplement and ten (10) study questions. The participant of interest, real or apparent, that is related to a specific educational activity. Any individual who should, in order, read the learning objectives contained at the beginning of this supplement, read neglects to provide information about relevant financial relationships will be disqualified from the supplement, answer all questions in the post test, and complete the evaluation form. -

Aesthetic Surgery of the Face Surgical Anatomy of the Face, SMAS, Facial Spaces and Retaining Ligaments 79
SECTION I Aesthetic Surgery of the Face Surgical anatomy of the face, SMAS, facial spaces and retaining ligaments 79 6 Anatomy of the aging face Bryan Mendelson and Chin-Ho Wong intraoperative map for the surgeons to safely navigate to the SYNOPSIS area of interest to correct aging changes. This is most impor- Aging of the face is a multifactorial process that can be explained tant in addressing the overriding concern, being the course of on an anatomical basis. the facial nerve branches. An anatomical approach to surgical The face is constructed of five basic layers that are bound together rejuvenation of the face provides the way to obtaining a by a system of facial retaining ligaments. “natural” result that is lasting and with minimal morbidity. Fig. 6.1 Regions of the face. The mobile anterior face is functionally adapted for To facilitate the mobility needed for facial expression independent facial expressions and is separated from the relatively fixed lateral face (shaded), of the basic functions of the face, particularly of mastication, a which overlies masticatory structures. A vertical line of retaining ligaments (red) separates the anterior and lateral face. These ligaments are, from above: temporal, series of soft tissue spaces are incorporated into the architecture Regions of the face lateral orbital, zygomatic, masseteric, and mandibular ligaments. In the anterior Fig. 6.2 The face is constructed of five basic layers. This five-layered construct of the face. face, the mid-cheek is split obliquely into two separate functional parts by the is most evident in the scalp but exists in the rest of the face, with significant This arrangement, most clearly seen in the scalp, also exists in mid-cheek groove (dotted line) related to two cavities: the periorbital part above modification and compaction for functional adaptation. -

Financial and Proprietary Interest: Nil Financial Support: Nil Eye (2006) 20, 1429–1430. Doi:10.1038/Sj.Eye.6702289; Published
Correspondence 1430 Financial and proprietary interest: Nil following the initial insult, he developed a secondary LE Financial support: Nil vitreous haemorrhage, which failed to improve with conservative management. Anticoagulation was optimal Eye (2006) 20, 1429–1430. doi:10.1038/sj.eye.6702289; throughout. He underwent a vitrectomy under local published online 17 February 2006 anaesthesia, leaving his LE visual acuity at 6/60 with resolved choroidal detachment, a flat retina, and no macular oedema. Sir, Bilateral choroidal detachments due to massive pulmonary embolism Comment Serous choroidal detachment involves the exudation of Choroidal detachment (in the absence of haemorrhage) is serum into the suprachoroidal space. There are several caused by the transudation of serum into the mechanisms for this, including low intraoccular pressure suprachoroidal space. This is most commonly due to an (IOP), which can be a result of ciliary body increase in orbital transmural pressure, because of hypoperfusion.1 We consider the most likely mechanism 1 trauma, serum exudation, or inflammation. To the best for this patient’s choroidal detachment to be a low of our knowledge, choroidal detachment is unreported in arterial perfusion pressure. Acute massive PE leads to an association with pulmonary embolism (PE). We report acute rise in pulmonary artery pressure. This leads to this previously serious occular complication of acute reduced pulmonary blood flow and impaired left heart massive PE. filling, leading to a low systemic blood pressure.2 The low cardiac output state leads to reduced carotid perfusion, and therefore reduced flow in the ophthalmic Case report An 86-year-old man presented following a collapse outside his general practioner (GP) surgery. -

Steriseal Ophthalmic Products
OPHTHALMICS The Products to be seen with Steriseal Ophthalmic Products Welcome to the latest Steriseal brochure, which we hope you not only find informative but also simple to use. The redesign of the brochure enables products to be selected by code, type or size by a simple indexing method. Covering the following procedures: • Cataract • Vitreo-retinal • Refractive • Lacrimal These products are available as single use sterile Steriseal branded items or, for kit packers, as non-sterile Steriseal branded items. Whilst the ownership of the company has now changed to Aspen Medical Europe Ltd., all of our products continue to be manufactured in the same high quality clean room environment, to the same international standards and the latest state of the art manufacturing technology. Therefore you can expect the same quality of product that has been available since the brand was introduced in 1977. Under Aspen Medical Europe Ltd. our investment into new products will continue across the whole range of ophthalmology disciplines. Our aim is to develop products that follow the emerging procedural trends, therefore do feel free to contact ourselves with any new ideas and or concepts you wish to discuss. Your contact point is: Tom Moss - Business Development Manager Tel +44 (0)1527 587709 E.mail [email protected] For our valued customers who wish to place orders or discuss any other aspect of our business your contact point is: Richard Allen - Customer Service Manager Tel +44 (0)1527 587728 E.mail [email protected] Aspen Medical Europe Ltd. Thornhill Road, North Moons Moat Redditch B98 9NL U.K. -

Safety of Primary Intraocular Lens Insertion in Unilateral Childhood Traumatic Cataract
ORIGINAL ARTICLE J Nepal Med Assoc 2008;47(172):179-85 Safety of Primary Intraocular Lens Insertion in Unilateral Childhood Traumatic Cataract Kumar S,1 Panda A,2 Badhu BP,3 Das H4 1Department of Ophthalmology, Subharti Institute of Medical Sciences, Meerut, UP, India, 2Dr. Rajendra Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences (AIIMS), New Delhi, India, 3Department of Ophthalmology, B P Koirala Institute of Health Sciences, Dharan, Nepal, 4Siliguri Lions Netralaya, Hill Cart Road, Siliguri, India ABSTRACT This study analyzes the results of cataract surgery with primary intraocular lens implantation in unilateral childhood traumatic cataract following penetrating trauma and its long term follow up. It is a hospital based study of 114 children (age 3-10 years) with unilateral traumatic cataract who underwent extracapsular cataract extraction/ lens aspiration with implantation of posterior chamber intraocular lens (IOL). Primary posterior capsulotomy (PPC) was performed in 57 eyes and the rest 57 were without PPC (NPPC). The patients were followed up at regular intervals for a period of 3 years. Postoperative inflammation and pupillary capture were two frequent complications seen during postoperative period. Development of posterior capsular opacification (PCO) was 1/57, 4/57 at 8th week and 7/30 and 14/39 at 6 months, in PPC and NPPC group, respectively. Best corrected visual acuity (BCVA) ≥ 6/18 was achieved in 50% of eyes at 8th week post operatively and the same at 3 years with/without membranectomy/capsulotomy was evident in 73.3% of eyes. Meticulous case selection with insersion of “in the bag IOL” and subjecting the traumatized cataractous eyes to primary posterior capsulotomy are factors responsible for optimal outcome in unilateral traumatic cataract in children.