Morphological Variability of Liza Abu (Heckel, 1843) from Northwestern Part of the Persian Gulf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Examining the Evolution of the Khuzestan Urban Population Using the Urban Primacy Indexes
INTERNATIONAL JOURNAL OF SCIENTIFIC & TECHNOLOGY RESEARCH VOLUME 6, ISSUE 12, DECEMBER 2017 ISSN 2277-8616 Examining The Evolution Of The Khuzestan Urban Population Using The Urban Primacy Indexes Mokhtar Karami, Rasol Sarvestan, Ali Mohammad Mansourzadeh Abstract: Examining the status of a city in the surrounding metropolitan area network not only helpful for Specialists to understand the ups and downs of city life and surrounding it ,but also can set the groundwork for hierarchical relationships settlements and planners for discipline the urban network is studied. Research to study the evolution of the urban population in Khuzestan province was conducted during the statistical period (1957-2012). The method is a descriptive and analytical study. To collect the data, in addition the study of literature, the Facts Sheet (the statistical yearbooks and census of population and housing censuses in all courses) has been used. Then to enter data and analysis it the Excel and Minitab software was used. Models used in this study are Ginsberg index, Urban Primacy Index, Two City Index, Four City Index, Mehta’s Four City Index, Moomav and Alwosabi. The results show that is balance between the parameters of the Urban Primacy Indexes in Khuzestan province since 1957 to 1977. The process of balancing continue and is destroy until the beginning of the Imposed war and the depletion of the population of cities and in 1987 the Urban Primacy Index reached its highest level and due to the problems of the war in Ahvaz it earns the highest the Urban Primacy Index. Since 1987, the Urban Primacy Index reduced and their balancing process continues until 2012 that this balancing process due to natural population growth since after 1997. -

An Investigation on the Systems of Collecting and Extracting Greywater from Surface Water and Wastewater
American Journal of Engineering and Applied Sciences Original Research Paper An Investigation on the Systems of Collecting and Extracting Greywater from Surface Water and Wastewater 1 2 3 4 Saeid Eslamian, Mohammad Abdolhoseini, Kaveh Ostad-Ali-Askari and Vijay P. Singh 1Department of Water Engineering, College of Agriculture, Isfahan University of Technology, Isfahan, 8415683111, Iran 2Department of Water Engineering, Gorgan University of Agricultural Sciences and Natural Resources, Gorgan, Iran 3Department of Water Engineering, College of Agriculture, Isfahan University of Technology, Isfahan, 8415683111, Iran 4Department of Biological and Agricultural Engineering and Zachry Department of Civil Engineering, Texas A and M University, 321 Scoates Hall, 2117 TAMU, College Station, Texas 77843-2117, USA Article history Abstract: Lack of water resources, on the one hand and degradation of Received: 19-05-2018 the quality of available resources; on the other hand, raise serious Revised: 07-07-2018 concerns about water supply. Limited water resources in some areas Accepted: 15-08-2018 necessitate planning for use of unconventional resources, such as: Corresponding Author: Unconventional and recyclable water resources, which should be used Mohammad Abdolhoseini optimally. Therefore, there is an increasing need for use of low-quality Department of Water water in areas where freshwater is scarce, in which case, Engineering, Gorgan University unconventional water resources (such as saline water, brackish water of Agricultural Sciences and and household wastewater) can be considered as valuable resources. Natural Resources, Gorgan, Iran The aim of this study was to investigate the possibility of water Email: extraction from household wastewater (greywater) and surface water mohammad.abdolhoseini24@g mail.com running in villages in Abadan and to simultaneously evaluate some water collection systems to find the best one in order to use these water resources as unconventional and recyclable water resources for various uses such as: Agriculture and irrigation of date palms. -

Mayors for Peace Member Cities 2021/10/01 平和首長会議 加盟都市リスト
Mayors for Peace Member Cities 2021/10/01 平和首長会議 加盟都市リスト ● Asia 4 Bangladesh 7 China アジア バングラデシュ 中国 1 Afghanistan 9 Khulna 6 Hangzhou アフガニスタン クルナ 杭州(ハンチォウ) 1 Herat 10 Kotwalipara 7 Wuhan ヘラート コタリパラ 武漢(ウハン) 2 Kabul 11 Meherpur 8 Cyprus カブール メヘルプール キプロス 3 Nili 12 Moulvibazar 1 Aglantzia ニリ モウロビバザール アグランツィア 2 Armenia 13 Narayanganj 2 Ammochostos (Famagusta) アルメニア ナラヤンガンジ アモコストス(ファマグスタ) 1 Yerevan 14 Narsingdi 3 Kyrenia エレバン ナールシンジ キレニア 3 Azerbaijan 15 Noapara 4 Kythrea アゼルバイジャン ノアパラ キシレア 1 Agdam 16 Patuakhali 5 Morphou アグダム(県) パトゥアカリ モルフー 2 Fuzuli 17 Rajshahi 9 Georgia フュズリ(県) ラージシャヒ ジョージア 3 Gubadli 18 Rangpur 1 Kutaisi クバドリ(県) ラングプール クタイシ 4 Jabrail Region 19 Swarupkati 2 Tbilisi ジャブライル(県) サルプカティ トビリシ 5 Kalbajar 20 Sylhet 10 India カルバジャル(県) シルヘット インド 6 Khocali 21 Tangail 1 Ahmedabad ホジャリ(県) タンガイル アーメダバード 7 Khojavend 22 Tongi 2 Bhopal ホジャヴェンド(県) トンギ ボパール 8 Lachin 5 Bhutan 3 Chandernagore ラチン(県) ブータン チャンダルナゴール 9 Shusha Region 1 Thimphu 4 Chandigarh シュシャ(県) ティンプー チャンディーガル 10 Zangilan Region 6 Cambodia 5 Chennai ザンギラン(県) カンボジア チェンナイ 4 Bangladesh 1 Ba Phnom 6 Cochin バングラデシュ バプノム コーチ(コーチン) 1 Bera 2 Phnom Penh 7 Delhi ベラ プノンペン デリー 2 Chapai Nawabganj 3 Siem Reap Province 8 Imphal チャパイ・ナワブガンジ シェムリアップ州 インパール 3 Chittagong 7 China 9 Kolkata チッタゴン 中国 コルカタ 4 Comilla 1 Beijing 10 Lucknow コミラ 北京(ペイチン) ラクノウ 5 Cox's Bazar 2 Chengdu 11 Mallappuzhassery コックスバザール 成都(チォントゥ) マラパザーサリー 6 Dhaka 3 Chongqing 12 Meerut ダッカ 重慶(チョンチン) メーラト 7 Gazipur 4 Dalian 13 Mumbai (Bombay) ガジプール 大連(タァリィェン) ムンバイ(旧ボンベイ) 8 Gopalpur 5 Fuzhou 14 Nagpur ゴパルプール 福州(フゥチォウ) ナーグプル 1/108 Pages -

Dr. Mehdi Mumipour C.V
Mehdi mumipur Assistant Professor College: Faculty of Marine Natural Resources Executive Activities Dr. Mehdi Mumipour C.V. (November 2018) Educational Background: High School Diploma: Empirical Sciences, Bekhradian High school - Behbahan, 1996 Bachelor of Science (BS): Geology, Shiraz University- Shiraz, Iran, 2002 Master of Science (MSc): Remote Sensing and Geographic Information System (GIS)- University of Tabriz –Tabriz, Iran, 2004 Ph.D.: Physical Geography – Geomorphology, University of Tabriz –Tabriz, Iran,2012 Master's thesis: Comparison of Two Soil Erosion Models (MPSIAC and RUSLE) Using Remote Sensing Data and GIS, Case Study of Ojan-Chay Sub-basin Ph.D. thesis: Analysis of the role of active tectonics in evolution of drainage networks - Case study of Dinarkooh area Full Knowlegde of remote sensing and GIS softwares: ArcGIS 10.4 and ENVI 5.1 Research records: Execution of 4 Academic Research Projects: "Extracting Geomorphometric Parameters from Digital Elevation Model (DEM) - A Case Study of Khayiz Region, Behbahan" "Study of Coastal Water Quality Using Satellite Images" "Investigation of Changes in Soil Salinity and its Relationship to dust storm using satellite images in the Abadan and Khorramshahr districts" “Comparison of ASTER and Landsat 8 OLI satellite images for detection of Carbonate and Clay minerals- case study of Anaran Anticline” Collaboration in 5 Academic Research Projects: "Estimating soil erosion using satellite images and GIS in the Abshirin Basin" "Preparation of a geological map using satellite ETM+ Landsat images and Geographic Information System (GIS)- Behbahan Case Study" "Simulation and Analysis of Relationships Between Rainfall-Runoff and Flood of Karun River Using HEC-RAS HEC-HMS Integrated Model and Remote Sensing" "Study of gardens of East Azarbaijan province using remote sensing technology" "Establishment of digital database of green space of Municipality of Tabriz region 8". -

Sociobiology 65(1): 24-30 (March, 2018) Special Issue DOI: 10.13102/Sociobiology.V65i1.1784
Sociobiology 65(1): 24-30 (March, 2018) Special Issue DOI: 10.13102/sociobiology.v65i1.1784 Sociobiology An international journal on social insects RESEARCH ARTICLE - TERMITES Termites of Iranian date palm orchards and their spatial and temporal distribution M Latifian1, B Rad1, B Habibpour2 1 - Agricultural Research, Education and Extension Organization (AREEO),Horticulture Research institute, Date Palm and Tropical Fruit Research Center, Ahvaz, Iran 2 - Department of Plant Protection, College of Agriculture, Shahid Chamran University of Ahvaz, Ahvaz, Iran Article History Abstract This study was conducted during 2013-2014 to determine the dominant species and Edited by behavior of termites foraging in date palm orchards in Khuzestan province, Iran. Paulo F. Cristaldo, UFS, Brazil Received 06 June 2017 Starting in early March, the ‘cluster random sampling’ method was used within sixty- Initial acceptance 28 July 2017 five sampling plots. Termite species were sampled by breaking open mud tubing and Final acceptance 13 August 2017 collecting different castes, then bringing them to the laboratory for identification Publication date 30 March 2018 using the scientific keys. Glass microscope slides of different body parts, especially the labium, maxillae, palps, mandibles, labrum, and clypeus. The Simpson Diversity Keywords Index was used to determine the level of dominance. Results showed three species Date palm, Iranian termites, temporal distribution, spatial distribution. of termites, Microcerotermes diversus Silvestri, Microcerotermes buettikeriChhotani and Bose and Amitermes vilis Hagen, all family Termitidae, are active in Khuzestan Corresponding author date palm plantations. Microcerotermes diversus was dominant with a correlation Masoud Latifian coefficient 0.997 and 8.87, respectively. Seasonal population fluctuations of workers, Horticulture Research institute nymphs, soldiers and winged castes had several peaks over the years. -

Mayors for Peace Gratefully Welcomes the Following New Members 49 New Cities Have Joined Mayors for Peace
Mayors for Peace Gratefully Welcomes the Following New Members 49 new cities have joined Mayors for Peace. Thus, the Conference involves 6,084 cities in 158 countries and regions around the world. As of June 1, 2014 No Country City/Municipality Mayor 1 Austria Amstetten Ms. Ursula Puchebner 2 Belarus Rogachev Mr. Sergey Vladimirovich Yakovlev 3 Germany Sömmerda Mr. Ralf Hauboldt 4 Iran Abhar Mr. Abdol-Ali Ghasempour 5 Iran Andimeshk Mr. Mohammad Sharifimoghadam 6 Iran Arsanjan Mr. Majid Ebrahimi 7 Iran Arvandkenar Mr. Yousof Deris 8 Iran Ashkezar Mr. Mohammad Ali Salmani 9 Iran Bagh Malek Mr. Hassan Nabovvati 10 Iran Beyza Mr. Yazdan Rahimi 11 Iran Dehaj Mr. Hamed Barzegari 12 Iran Emamzadeh Abdollah (Mazandaran) Mr. Mahdi Fatemi 13 Iran Esfarayen Mr. Hadi Zarei 14 Iran Farashband Mr. Mohammad Reza Noshadi 15 Iran Fasa Mr. Rasoul Haghighi 16 Iran Fooladshahr Mr. Hossein Amiri 17 Iran Ghir Mr. Mansour Salehi 18 Iran Golzar Mr. Gholamreza Said 19 Iran Hendijan Mr. Gholam-Reza Darvishi 20 Iran Izeh Mr. Mehran Babapour 21 Iran Kazemabad Mr. Farhad Ebrahimpour 22 Iran Khatoon Abad Mr. Mohammad Ali Niktabe 23 Iran Khorsand Mr. Hassan Riyahian 24 Iran Konartakhteh Mr. Reza Nowzari 25 Iran Kouhanjan Mr. Ahmad Reza Bagheri 26 Iran Laleh Zar (Kerman) Mr. Javad Khanamani 27 Iran Mardehak Mr. Nader Paye-Dar 28 Iran Meymand Mr. Ashghar Karimi 29 Iran Najaf Shahr Mr. Ali Najafabadipour 30 Iran Nezam Shahr Mr. Mohammadreza Mohseninia 31 Iran Orzueeyeh Mr. Reza Golsamanpour 32 Iran Rabor Mr. Karim Ghasemi 33 Iran Reyhan Shahr Mr. Gholam-Hossein Dini 34 Iran Reyne Larijan Mr. -

River Water Quality Zoning: a Case Study of Karoon and Dez River System
Iranian J Env Health Sci Eng, 2004, Vol.1, No. 2, pp.16-27 Mohammad Karamou et al: River Water Quality… River Water Quality Zoning: A Case Study of Karoon and Dez River System *M Karamouz 1, N Mahjouri 2, R Kerachian 3 1School of Civil and Environmental Engineering, Amirkabir University, Iran 2 Faculty of Environmental Engineering, University of Tehran, Iran 3Dept. of Civil Engineering, University of Tehran, Iran ABSTRACT Karoon-Dez River basin, with an area of 67000 square kilometers, is located in southern part of Iran. This river system supplies the water demands of 16 cities, several villages, thousands hectares of agricultural lands, and several hydropower plants. The increasing water demands at the project development stage including agricultural networks, fish hatchery projects, and inter-basin water transfers, have caused a gloomy future for water quality of the Karoon and Dez Rivers. A good part of used agricultural water, which is about 8040 million cubic meters, is returned to the rivers through agricultural drainage systems or as non-point, return flows. River water quality zoning could provide essential information for developing river water quality management policies. In this paper, a methodology is presented for this purpose using methods of c -mean crisp classification and a fuzzy clustering scheme. The efficiency of these clustering methods was evaluated using water quality data gathered from the monitoring sampling points along Karoon and Dez Rivers. The results show that the proposed methodology can provide valuable information to support decision-making and to help river water quality management in the region. Keywords: Fuzzy Clustering, Data classification, Water quality zoning, River systems, Karoon, Dez INTRODUCTION water quality control projects. -
Curriculum Vitae 2) Scientific and Administrative Positions 4
Curriculum Vitae 1) Personal Full Name: Navid Khaiat Profession: Geotechnical Engineering Nationality: Iran Date of Birth: 6 July, 1978 Place of Birth: Khoramshahr, Iran. Address: Golestan Bolvar, Farhangshahr Square, Islamic Azad University, Ahvaz Branch. Postcode: 1915, Post box 61349-37333 E-mail: 1) [email protected] 2) [email protected] Phone number: +986113348354 Mobil number: +989166158266 2) Scientific and Administrative Positions Assistant professor, Grad 7-full-time academic member in Islamic Azad University, Ahvaz branch, since 2004. Head of undergraduate department in Civil Engineering., Islamic Azad University, Ahvaz branch. 2010 – 2012. Member of Research Council in Islamic Azad University, Ahvaz since 2010. Observer and member of control group in geotechnical engineering organization of Khuzestan, since 1386. 3) Education: 1- PhD in Civil Engineering, Geotechnical Engineering. University of Islamic Azad, Tehran Science and Research Branch. Tehran, Iran. 2004-2010. 2- M.Sc. in Civil Engineering, Shahid Chamran University 2001-2003. 3- B.Sc. in Civil Engineering, Shahid Chamran University, Ahvaz, Iran. 1996-2000 4) Research and laboratory background 4-1) PhD thesis title: Investigation in the effect of inherent and induced anisotropy on the undrained behavior on Silt-Sand mixture Supervisor: Dr. Abbas Ghalandarzadeh, A, Associate professor in Tehran University Advisor: Dr. Mohammad Kazem Jaefari, professor of International Institute of Earthquake of Iran, Geotechnical faculty 1 4-2) M.Sc. thesis title: study of Shear strength properties of mixtures of bentonite-sand Supervisor: Dr. Mohammad Sirus Pakbaz, Associate professor in Shahid Chamran University, Ahvaz Advisor: Dr. Reza ebn Jalal, Assistant professor in Shahid Chamran University, Ahvaz 5) Experience working with laboratory equipment : Conducting over 150 experiments using Hollow Cylinder Torsion Shear Tests during the PhD thesis. -

Iran Ulaşim Altyapisi Ve Gümrük Kapilari
T.C. Tahran Büyükelçiliği Ticaret Müşavirliği 26/09/2019 İRAN ULAŞIM ALTYAPISI VE GÜMRÜK KAPILARI Not: Bu nottaki bilgilerin, yürürlükteki İran’a yönelik yaptırımlar gözönüne alınarak değerlendirilmesi gerekmektedir. 1. Ticari Deniz Limanları 1.1. Limanların Genel Değerlendirmesi İran Gümrük İdaresi (IRICA) tarafından yapılan ve Abadan, Imam Khomeini, Bushehr, Khorramshahr, Genaveh, Lengeh, Chavibdeh, Arvandkenar, Charak, Dayyer, Shahid Rajaee, Shahid Bahonar, Qeshm, Tiab, Jask, Chabahar, Fereydounkenar, Noshahr, Astara, Amirabad ve Anzali olmak üzere 21 limanı kapsayan çalışmada, ülke limanlarında günde 200 geminin yükleme ve boşaltma yaptığı, limanlardan 6,7 milyon ton ürünün de transit ticaretinin yapıldığı açıklanmıştır. Aynı açıklamada, İran’ın 11 büyük limanında kapasite kullanımının %20’de kaldığı belirtilmiştir. İran limanlarında yılda ortalama 130 milyon ton civarı yükleme ve boşaltma yapılmaktadır. Bunun 90 milyon ton civarı (63 milyon ton ihracat; 27 milyon ton ithalat) petrol dışı ürünler iken, 40 milyon civarı ise petrol ve petrol ürünleridir. İran’ın ticaret limanları üç grupta (Hazar Denizi kıyısında bulunan limanlar, Basra Körfezi’ndeki limanlar, Hint Okyanusu’ndaki limanlar) ele alınabilir. Tablo 1: Ticari Deniz Limanları Limanın Adı Boylam (°) Enlem (°) Liman Kapasitesi, Ton/Yıl Shahid Rajaee, Bandar Abbas 56.2666667 27.1833333 70.000.000 Imam Khomeini 49.1055556 30.4355556 50.000.000 Bandar-e Mahshahr 30.4678708 49.1723829 40.000.000 Bushehr 50.8333333 28.9666667 20.000.000 Asaluyeh 52.6166667 27.4666667 18.000.000 Chabahar 60.6300000 25.2866667 15.000.000 Shahid Bahonar 56.2091237 27.1520753 10.000.000 Amirabad 48.9899790 30.5769211 4.000.000 Abadan 48.9899790 30.5769211 4.000.000 Khorramshahr 48.2269563 30.3641484 3.000.000 Lengeh 54.8805556 26.5580556 840.000 1.2. -

Academia Arena 2016;8(7)
Academia Arena 2016;8(7) http://www.sciencepub.net/academia Survey of palm grovesposition in rural community economic (case study: villages of Abadan & Arvand Kenar) Hamid Khalafi1, Dr. Eynollah Khalili2 1. Department of Geography And Rural Planning, Ahvaz Branch, Islamic Azad University, Ahvaz, Iran 2. Department of Geography And Rural Planning, Assistant Professor, Ahvaz Branch, Islamic Azad University, Ahvaz, Iran Abstract: The purpose of this study was to investigate the palm groves of rural Draqtsadjamh (Case Study of Rural Abadan and Arvandkenar), which was conducted in 1392. The aim of applied research is descriptive survey was conducted. The main tool for data collection, questionnaires and interviews, their livelihood, simple charts the data obtained were analyzed. Results showed that the mean area under cultivation in the region dates for each beneficiary 6/1 Hktarv data types include: Astmrvn, Barhi, Gantar, Balyânî, Fersi, Zahedi, Khazravi, and Berim you. According to data on Sayer dates Nkhldaran date production is the most important figure. The findings also showed that the development of palm groves can be effective on Drbalabrdn the livelihoods of rural communities, as well as reducing migration from rural to urban society can be obtained from the current income of the sample (about 22 million USD per yearcould be one of these sources of income. [Hamid Khalafi, Eynollah Khalili. Survey of palm grovesposition in rural community economic (case study:villages of Abadan & Arvand Kenar). Academ Arena 2016;8(7):20-25]. ISSN 1553-992X (print); ISSN 2158-771X (online). http://www.sciencepub.net/academia. 5. doi:10.7537/marsaaj080716.05. Key words: Groves, business center, the rural economy. -

Iranian Nuclear Politics
05/64 Iranian Nuclear Politics Conflict Studies Research Centre Middle East Series 05/64 1 Iranian Nuclear Politics: The International Dimensions Dr Babak Ganji Key Points * The nuclear policy of the Ahmadinezhad government should be analysed within the framework of the grand strategy of the Iranian state which is aimed at challenging the US in the Persian Gulf and south Asia in particular. * Since the advent of the new government, cleavages within the Iranian state apparatus on the nuclear issue have also changed. The radical-conservative camp, most notably Iran’s supreme leader Ayatollah Ali Khamene’i, would prefer Iran to pursue industrial-scale uranium enrichment within the framework of the nuclear Non-Proliferation Treaty. There are serious differences of opinion between advocates of nuclear opacity such as Ahmad Tavakkoli, who have called for the continuation of talks with the International Atomic Energy Agency, and those such as the secretary of the Supreme National Security Council Ali Larijani who seek to tighten Iran’s relations with Russia, China, India and Pakistan. In the centre right and reformist camp, some still favour talks with the EU, although there have been calls for withdrawal from the NPT if the Iranian case is referred to the UN Security Council. The Majlis, particularly the National Security and Foreign Policy Committee, is still playing an important role in the nuclear debate. * There are serious differences of opinion over grand strategy within the radical and conservative camps, usually expressed in terms of the wisdom of Iranian efforts to bring Russia and China into the talks. A powerful current within the state apparatus, strongly believes in self-reliance, contending that neither Russia nor China will sacrifice their national interests for Iran. -
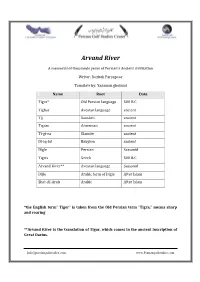
Arvand River
Arvand River A memorial of thousands years of Persian’s Ancient civilization Writer: Rozbeh Parsapoor Translate by: Yasaman gholami Name Root Date Tigra* Old Persian language 500 B.C Tighar Avestan language ancient Tjj Sanskrit ancient Tigran Armenian ancient Ti‐gi‐ra Elamite ancient Di‐iq‐lat Babylon ancient Digle Persian Sassanid Tigris Greek 500 B.C Arvand River** Avestan language Sassanid Dijle Arabic form of Digle After Islam Shat‐Al‐Arab Arabic After Islam *the English term” Tiger” is taken from the Old Persian term "Tigra," means sharp and roaring **Arvand River is the translation of Tigar, which comes in the ancient inscription of Great Darius. [email protected] www.Persiangulfstudies.com Abstract: Arvand River is one of the Iran‐Iraq Border Rivers, which is located in Khorramshahr and Abadan. The length of its border is 84 kilometers. Arvand River constitutes of meeting of Tigris and Euphrates rivers in al‐Qurnah, which is in 110 kMs north of Abadan. It passes from southeast of Basra, Iraq, Khorramshahr and Abadan and then in 8 kMs south of Arvandkenar joined to the Persian Gulf (Pars Sea). Unfortunately, Arabs of south of Persian Gulf had a long history in changing and faking the historical names and universal documents, thus the ancient name of Arvand River, which is related to 2500 years ago, is not accepted by them, and they called it Shatt‐al‐Arab, which has existed for 1400years. However, Arvand River, which is mentioned in the Shahnama and classical texts, is known in all formal correspondence in Iran. Arvand River is officially recognized in the Islamic consultative assembly in Iran as well.