Aging and Rejuvenation of Neural Stem Cells and Their Niches
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neuregulin 1–Erbb2 Signaling Is Required for the Establishment of Radial Glia and Their Transformation Into Astrocytes in Cerebral Cortex
Neuregulin 1–erbB2 signaling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex Ralf S. Schmid*, Barbara McGrath*, Bridget E. Berechid†, Becky Boyles*, Mark Marchionni‡, Nenad Sˇ estan†, and Eva S. Anton*§ *University of North Carolina Neuroscience Center and Department of Cell and Molecular Physiology, University of North Carolina School of Medicine, Chapel Hill, NC 27599; †Department of Neurobiology, Yale University School of Medicine, New Haven, CT 06510; and ‡CeNes Pharamceuticals, Inc., Norwood, MA 02062 Communicated by Pasko Rakic, Yale University School of Medicine, New Haven, CT, January 27, 2003 (received for review December 12, 2002) Radial glial cells and astrocytes function to support the construction mine whether NRG-1-mediated signaling is involved in radial and maintenance, respectively, of the cerebral cortex. However, the glial cell development and differentiation in the cerebral cortex. mechanisms that determine how radial glial cells are established, We show that NRG-1 signaling, involving erbB2, may act in maintained, and transformed into astrocytes in the cerebral cortex are concert with Notch signaling to exert a critical influence in the not well understood. Here, we show that neuregulin-1 (NRG-1) exerts establishment, maintenance, and appropriate transformation of a critical role in the establishment of radial glial cells. Radial glial cell radial glial cells in cerebral cortex. generation is significantly impaired in NRG mutants, and this defect can be rescued by exogenous NRG-1. Down-regulation of expression Materials and Methods and activity of erbB2, a member of the NRG-1 receptor complex, leads Clonal Analysis to Study NRG’s Role in the Initial Establishment of to the transformation of radial glial cells into astrocytes. -

The Act of Controlling Adult Stem Cell Dynamics: Insights from Animal Models
biomolecules Review The Act of Controlling Adult Stem Cell Dynamics: Insights from Animal Models Meera Krishnan 1, Sahil Kumar 1, Luis Johnson Kangale 2,3 , Eric Ghigo 3,4 and Prasad Abnave 1,* 1 Regional Centre for Biotechnology, NCR Biotech Science Cluster, 3rd Milestone, Gurgaon-Faridabad Ex-pressway, Faridabad 121001, India; [email protected] (M.K.); [email protected] (S.K.) 2 IRD, AP-HM, SSA, VITROME, Aix-Marseille University, 13385 Marseille, France; [email protected] 3 Institut Hospitalo Universitaire Méditerranée Infection, 13385 Marseille, France; [email protected] 4 TechnoJouvence, 13385 Marseille, France * Correspondence: [email protected] Abstract: Adult stem cells (ASCs) are the undifferentiated cells that possess self-renewal and differ- entiation abilities. They are present in all major organ systems of the body and are uniquely reserved there during development for tissue maintenance during homeostasis, injury, and infection. They do so by promptly modulating the dynamics of proliferation, differentiation, survival, and migration. Any imbalance in these processes may result in regeneration failure or developing cancer. Hence, the dynamics of these various behaviors of ASCs need to always be precisely controlled. Several genetic and epigenetic factors have been demonstrated to be involved in tightly regulating the proliferation, differentiation, and self-renewal of ASCs. Understanding these mechanisms is of great importance, given the role of stem cells in regenerative medicine. Investigations on various animal models have played a significant part in enriching our knowledge and giving In Vivo in-sight into such ASCs regulatory mechanisms. In this review, we have discussed the recent In Vivo studies demonstrating the role of various genetic factors in regulating dynamics of different ASCs viz. -

Regulation of Adult Neurogenesis in Mammalian Brain
International Journal of Molecular Sciences Review Regulation of Adult Neurogenesis in Mammalian Brain 1,2, 3, 3,4 Maria Victoria Niklison-Chirou y, Massimiliano Agostini y, Ivano Amelio and Gerry Melino 3,* 1 Centre for Therapeutic Innovation (CTI-Bath), Department of Pharmacy & Pharmacology, University of Bath, Bath BA2 7AY, UK; [email protected] 2 Blizard Institute of Cell and Molecular Science, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London E1 2AT, UK 3 Department of Experimental Medicine, TOR, University of Rome “Tor Vergata”, 00133 Rome, Italy; [email protected] (M.A.); [email protected] (I.A.) 4 School of Life Sciences, University of Nottingham, Nottingham NG7 2HU, UK * Correspondence: [email protected] These authors contributed equally to this work. y Received: 18 May 2020; Accepted: 7 July 2020; Published: 9 July 2020 Abstract: Adult neurogenesis is a multistage process by which neurons are generated and integrated into existing neuronal circuits. In the adult brain, neurogenesis is mainly localized in two specialized niches, the subgranular zone (SGZ) of the dentate gyrus and the subventricular zone (SVZ) adjacent to the lateral ventricles. Neurogenesis plays a fundamental role in postnatal brain, where it is required for neuronal plasticity. Moreover, perturbation of adult neurogenesis contributes to several human diseases, including cognitive impairment and neurodegenerative diseases. The interplay between extrinsic and intrinsic factors is fundamental in regulating neurogenesis. Over the past decades, several studies on intrinsic pathways, including transcription factors, have highlighted their fundamental role in regulating every stage of neurogenesis. However, it is likely that transcriptional regulation is part of a more sophisticated regulatory network, which includes epigenetic modifications, non-coding RNAs and metabolic pathways. -

Rapid and Efficient Generation of Oligodendrocytes from Human
Rapid and efficient generation of oligodendrocytes PNAS PLUS from human induced pluripotent stem cells using transcription factors Marc Ehrlicha,b, Sabah Mozafaric,d,e,f, Michael Glatzab, Laura Starosta,b, Sergiy Velychkob, Anna-Lena Hallmanna,b, Qiao-Ling Cuig, Axel Schambachh, Kee-Pyo Kimb, Corinne Bachelinc,d,e,f, Antoine Marteync,d,e,f, Gunnar Hargusa,b, Radia Marie Johnsoni, Jack Antelg, Jared Sterneckertj, Holm Zaehresb,k, Hans R. Schölerb,l, Anne Baron-Van Evercoorenc,d,e,f, and Tanja Kuhlmanna,1 aInstitute of Neuropathology, University Hospital Münster, 48149 Muenster, Germany; bDepartment of Cell and Developmental Biology, Max Planck Institute for Molecular Biomedicine, 48149 Muenster, Germany; cINSERM, U1127, F-75013 Paris, France; dCNRS, UMR 7225, F-75013 Paris, France; eSorbonne Universités, Université Pierre et Marie Curie Paris 06, UM-75, F-75005 Paris, France; fInstitut du Cerveau et de la Moelle epinière-Groupe Hospitalier Pitié-Salpêtrière, F-75013 Paris, France; gMontreal Neurological Institute, McGill University, Montreal, QC, Canada H3A 2B4; hInstitute of Experimental Hematology, Hannover Medical School, 30625 Hannover, Germany; iDepartment of Physiology, McGill University, Montreal, QC, Canada H3A 2B4; jDFG Research Center for Regenerative Therapies, Technische Universität Dresden, 01307 Dresden, Germany; kMedical Faculty, Department of Anatomy and Molecular Embryology, Ruhr-University Bochum, 44801 Bochum, Germany; and lMedicial Faculty, Westphalian Wilhelms-University of Muenster, 48149 Muenster, Germany Edited by Brigid L. M. Hogan, Duke University Medical Center, Durham, NC, and approved February 1, 2017 (received for review August 30, 2016) Rapid and efficient protocols to generate oligodendrocytes (OL) more, these protocols require long culture periods (70–150 d) to + from human induced pluripotent stem cells (iPSC) are currently obtain O4 OL and show limited efficiency (9–12). -

Differential Timing and Coordination of Neurogenesis and Astrogenesis
brain sciences Article Differential Timing and Coordination of Neurogenesis and Astrogenesis in Developing Mouse Hippocampal Subregions Allison M. Bond 1, Daniel A. Berg 1, Stephanie Lee 1, Alan S. Garcia-Epelboim 1, Vijay S. Adusumilli 1, Guo-li Ming 1,2,3,4 and Hongjun Song 1,2,3,5,* 1 Department of Neuroscience and Mahoney Institute for Neurosciences, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA; [email protected] (A.M.B.); [email protected] (D.A.B.); [email protected] (S.L.); [email protected] (A.S.G.-E.); [email protected] (V.S.A.); [email protected] (G.-l.M.) 2 Department of Cell and Developmental Biology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA 3 Institute for Regenerative Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA 4 Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA 5 The Epigenetics Institute, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19104, USA * Correspondence: [email protected] Received: 19 October 2020; Accepted: 24 November 2020; Published: 26 November 2020 Abstract: Neocortical development has been extensively studied and therefore is the basis of our understanding of mammalian brain development. One fundamental principle of neocortical development is that neurogenesis and gliogenesis are temporally segregated processes. However, it is unclear how neurogenesis and gliogenesis are coordinated in non-neocortical regions of the cerebral cortex, such as the hippocampus, also known as the archicortex. Here, we show that the timing of neurogenesis and astrogenesis in the Cornu Ammonis (CA) 1 and CA3 regions of mouse hippocampus mirrors that of the neocortex; neurogenesis occurs embryonically, followed by astrogenesis during early postnatal development. -

NEUROGENESIS in the ADULT BRAIN: New Strategies for Central Nervous System Diseases
7 Jan 2004 14:25 AR AR204-PA44-17.tex AR204-PA44-17.sgm LaTeX2e(2002/01/18) P1: GCE 10.1146/annurev.pharmtox.44.101802.121631 Annu. Rev. Pharmacol. Toxicol. 2004. 44:399–421 doi: 10.1146/annurev.pharmtox.44.101802.121631 Copyright c 2004 by Annual Reviews. All rights reserved First published online as a Review in Advance on August 28, 2003 NEUROGENESIS IN THE ADULT BRAIN: New Strategies for Central Nervous System Diseases ,1 ,2 D. Chichung Lie, Hongjun Song, Sophia A. Colamarino,1 Guo-li Ming,2 and Fred H. Gage1 1Laboratory of Genetics, The Salk Institute, La Jolla, California 92037; email: [email protected], [email protected], [email protected] 2Institute for Cell Engineering, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, Maryland 21287; email: [email protected], [email protected] Key Words adult neural stem cells, regeneration, recruitment, cell replacement, therapy ■ Abstract New cells are continuously generated from immature proliferating cells throughout adulthood in many organs, thereby contributing to the integrity of the tissue under physiological conditions and to repair following injury. In contrast, repair mechanisms in the adult central nervous system (CNS) have long been thought to be very limited. However, recent findings have clearly demonstrated that in restricted areas of the mammalian brain, new functional neurons are constantly generated from neural stem cells throughout life. Moreover, stem cells with the potential to give rise to new neurons reside in many different regions of the adult CNS. These findings raise the possibility that endogenous neural stem cells can be mobilized to replace dying neurons in neurodegenerative diseases. -

Neurogenesis in the Adult Brain
The Journal of Neuroscience, February 1, 2002, 22(3):612–613 Neurogenesis in the Adult Brain Fred H. Gage Laboratory of Genetics, The Salk Institute, La Jolla, California 92037 A milestone is marked in our understanding of the brain with the (Palmer et al., 1995; Shihabuddin et al., 2000), even where no recent acceptance, contrary to early dogma, that the adult ner- neurons existed (Palmer et al., 1999; Kondo and Raff, 2000). vous system can generate new neurons. One could wonder how These studies demonstrating that cells with stem cell properties this dogma originally came about, particularly because all organ- exist in the adult brain were conducted in vitro, so it is not clear isms have some cells that continue to divide, adding to the size of whether these cultured cells have the same potential in vivo as in the organism and repairing damage. All mammals have replicat- vitro. Nevertheless, they showed that we no longer had to consider ing cells in many organs and in some cases, notably the blood, that a complex neuron was required to divide for adult neuro- skin, and gut, stem cells have been shown to exist throughout life, genesis to occur. Now we know that these neurons can be gener- contributing to rapid cell replacement. Furthermore, insects, fish, ated from primitive cells, similar to what happens in and amphibia can replicate neural cells throughout life. An ex- development. ception to this rule of self-repair and continued growth was A second change in thinking was more gradual and conceptual, thought to be the mammalian brain and spinal cord. -
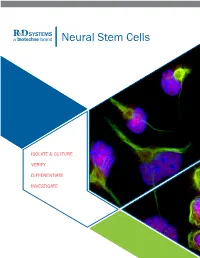
Neural Stem Cells
RnDSy-lu-2945 Neural Stem Cells ISOLATE & CULTURE VERIFY DIFFERENTIATE INVESTIGATE ISOLATE AND CULTURE Neural Stem cells (NSCs) require specialized media and growth factors to ensure efficient expansion. In addition to multipotent mouse and rat primary cortical stem cells, Bio-Techne offers a variety of serum-free neural media supplements, growth factors, and small molecules to maintain and expand NSCs. N21-MAX Cell Culture Supplements and Substrates • Improved Cell Health: high-quality culture reagents to ensure better growth and differentiation • Efficient Growth: optimized to enhance neural cell growth in culture Product Catalog # N-2 Plus Media Supplement AR003 N-2 MAX Media Supplement AR009 N21-MAX Media Supplement AR008 N21-MAX Insulin Free Media Supplement AR010 Competitor N21-MAX Vitamin A Free Media Supplement AR012 Holo-Transferrin 2914-HT Human Fibronectin, CF 1918-FN Bovine Fibronectin, CF 1030-FN Recombinant Human Fibronectin Full, CF 4305-FN Recombinant Human Fibronectin Fragment 2 3225-FN Recombinant Human Fibronectin Fragment 3 3938-FN Recombinant Human Fibronectin Fragment 4 3624-FN Recombinant Human Fibronectin, GMP 4305-GMP Recombinant Human Fibronectin, ACFP ACFP4305 Increased Synaptic Puncta and Neurite Outgrowth of ® Cultrex Poly-L-Lysine 3438-100-01 Primary Neurons Cultured in N21-MAX. E18 rat hippo- campal neurons were grown for 21 days in vitro in media supplemented with either N21-MAX Media Supplement (Catalog # AR008) or the neural media supplement from the most widely-used competitor. Staining for Synaptotagmin (yellow) showed more robust synaptic puncta and increased neurite outgrowth in neurons cultured in N21-MAX compared to those cultured in competitor media. Cells were stained with a Mouse Anti-Rat Synaptotagmin-1 Monoclonal Antibody (Catalog # MAB4364) followed by the NorthernLights™ (NL)557-conjugated Donkey Anti-Mouse IgG Secondary Antibody (Catalog # NL007). -

Neurogenesis in the Adult Human Hippocampus
1998 Nature America Inc. • http://medicine.nature.com ARTICLES Neurogenesis in the adult human hippocampus PETER S. ERIKSSON1,4, EKATERINA PERFILIEVA1, THOMAS BJÖRK-ERIKSSON2, ANN-MARIE ALBORN1, CLAES NORDBORG3, DANIEL A. PETERSON4 & FRED H. GAGE4 Department of Clinical Neuroscience, Institute of Neurology1, Department of Oncology2, Department of Pathology3, Sahlgrenska University Hospital, 41345 Göteborg, Sweden 4Laboratory of Genetics, The Salk Institute for Biological Studies, 10010 North Torrey Pines Road, La Jolla, California 92037, USA Correspondence should be addressed to F.H.G. The genesis of new cells, including neurons, in the adult human brain has not yet been demon- strated. This study was undertaken to investigate whether neurogenesis occurs in the adult human brain, in regions previously identified as neurogenic in adult rodents and monkeys. Human brain tissue was obtained postmortem from patients who had been treated with the thymidine analog, bromodeoxyuridine (BrdU), that labels DNA during the S phase. Using im- munofluorescent labeling for BrdU and for one of the neuronal markers, NeuN, calbindin or neu- ron specific enolase (NSE), we demonstrate that new neurons, as defined by these markers, are generated from dividing progenitor cells in the dentate gyrus of adult humans. Our results further indicate that the human hippocampus retains its ability to generate neurons throughout life. Loss of neurons is thought to be irreversible in the adult human one intravenous infusion (250 mg; 2.5 mg/ml, 100 ml) of bro- brain, because dying neurons cannot be replaced. This inability modeoxyuridine (BrdU) for diagnostic purposes11. One patient to generate replacement cells is thought to be an important diagnosed with a similar type and location of cancer, but with- http://medicine.nature.com cause of neurological disease and impairment. -

Stem Cells in Human Neurodegenerative Disorders — Time for Clinical Translation? Olle Lindvall1,2 and Zaal Kokaia2,3
Review series Stem cells in human neurodegenerative disorders — time for clinical translation? Olle Lindvall1,2 and Zaal Kokaia2,3 1Laboratory of Neurogenesis and Cell Therapy, Wallenberg Neuroscience Center, University Hospital, Lund, Sweden. 2Lund Stem Cell Center, Lund, Sweden. 3Laboratory of Neural Stem Cell Biology and Therapy, University Hospital, Lund, Sweden. Stem cell–based approaches have received much hype as potential treatments for neurodegenerative disorders. Indeed, transplantation of stem cells or their derivatives in animal models of neurodegenerative diseases can improve function by replacing the lost neurons and glial cells and by mediating remyelination, trophic actions, and modulation of inflammation. Endogenous neural stem cells are also potential therapeutic targets because they pro- duce neurons and glial cells in response to injury and could be affected by the degenerative process. As we discuss here, however, significant hurdles remain before these findings can be responsibly translated to novel therapies. In particular, we need to better understand the mechanisms of action of stem cells after transplantation and learn how to control stem cell proliferation, survival, migration, and differentiation in the pathological environment. Introduction patients need to be established, and all the associated ethical, Neurodegenerative disease is a term used for a wide range of regulatory, societal, and economical issues need to be addressed. acute and chronic conditions in which neurons and glial cells in Here, we discuss some general issues relating to the clinical trans- the brain and spinal cord are lost. In acute cases, for example, in lation of stem cells. We also describe how far stem cell–based response to ischemic stroke or spinal cord injury, different types approaches for treating some acute and chronic neurodegenera- of neurons and glial cells die within a restricted brain area over a tive disorders have advanced and define the critical milestones short time period. -

Endogenous Neurogenesis Replaces Oligodendrocytes and Astrocytes After Primate Spinal Cord Injury
The Journal of Neuroscience, February 22, 2006 • 26(8):2157–2166 • 2157 Development/Plasticity/Repair Endogenous Neurogenesis Replaces Oligodendrocytes and Astrocytes after Primate Spinal Cord Injury Hong Yang,1 Paul Lu,1 Heather M. McKay,2 Tim Bernot,2 Hans Keirstead,3 Oswald Steward,3 Fred H. Gage,4 V. Reggie Edgerton,5 and Mark H. Tuszynski1,6 1Department of Neurosciences, University of California, San Diego, La Jolla, California 92093-0626, 2California National Primate Research Center, University of California, Davis, California 95616, 3Department of Anatomy and Neurobiology, University of California, Irvine, California 92697, 4Laboratory of Genetics, Salk Institute, La Jolla, California 92037, 5Department of Physiological Science, University of California, Los Angeles, California 90095, and 6Veterans Administration Medical Center, San Diego, California 92161 Neurogenesis has been described in various regions of the CNS throughout life. We examined the extent of natural cell division and replacement from 7 weeks to 7 months after cervical spinal cord injury in four adult rhesus monkeys. Bromodeoxyuridine (BrdU) injections revealed an increase of Ͼ80-fold in the number of newly divided cells in the primate spinal cord after injury, with an average of 725,000 BrdU-labeled cells identified per monkey in the immediate injury zone. By 7 months after injury, 15% of these new cells expressed mature markers of oligodendrocytes and 12% expressed mature astrocytic markers. Newly born oligodendrocytes were present in zones of injury-induced demyelination and appeared to ensheath or remyelinate host axons. Thus, cell replacement is an extensive natural compensatory response to injury in the primate spinal cord that contributes to neural repair and is a potential target for therapeutic enhancement. -

Adult Neurogenesis, Mental Health, and Mental Illness: Hope Or Hype?
The Journal of Neuroscience, November 12, 2008 • 28(46):11785–11791 • 11785 Mini-Symposium Adult Neurogenesis, Mental Health, and Mental Illness: Hope or Hype? Amelia J. Eisch,1 Heather A. Cameron,2 Juan M. Encinas,3 Leslie A. Meltzer,4 Guo-Li Ming,5 and Linda S. Overstreet-Wadiche6 1Department of Psychiatry, University of Texas Southwestern Medical Center, Dallas, Texas 75390-9070, 2Unit on Neuroplasticity, Mood and Anxiety Disorders Program, National Institute of Mental Health, National Institutes of Health, Bethesda, Maryland 20892, 3Cold Spring Harbor Laboratory, Cold Spring Harbor, New York 11724, 4Department of Bioengineering, Stanford University, Stanford, California 94305-5435, 5Johns Hopkins University, Departments of Neurology and Neuroscience, Baltimore, Maryland 21205, and 6Department of Neurobiology, University of Alabama at Birmingham, Birmingham, Alabama 35294 Psychiatric and neurologic disorders take an enormous toll on society. Alleviating the devastating symptoms and consequences of neuropsychiatric disorders such as addiction, depression, epilepsy, and schizophrenia is a main force driving clinical and basic research- ersalike.Byelucidatingthesediseaseneuromechanisms,researchershopetobetterdefinetreatmentsandpreventivetherapies.Research suggests that regulation of adult hippocampal neurogenesis represents a promising approach to treating and perhaps preventing mental illness. Here we appraise the role of adult hippocampal neurogenesis in major psychiatric and neurologic disorders within the essential framework of recent