20. Long-Term Patient Care and Maintenance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Looking Back Featured Stories from the Past
ISSN-1079-7832 A Publication of the Immortalist Society Longevity Through Technology Volume 49 - Number 01 Looking Back Featured stories from the past www.immortalistsociety.org www.cryonics.org www.americancryonics.org Who will be there for YOU? Don’t wait to make your plans. Your life may depend on it. Suspended Animation fields teams of specially trained cardio-thoracic surgeons, cardiac perfusionists and other medical professionals with state-of-the-art equipment to provide stabilization care for Cryonics Institute members in the continental U.S. Cryonics Institute members can contract with Suspended Animation for comprehensive standby, stabilization and transport services using life insurance or other payment options. Speak to a nurse today about how to sign up. Call 1-949-482-2150 or email [email protected] MKMCAD160206 216 605.83A SuspendAnim_Ad_1115.indd 1 11/12/15 4:42 PM Why should You join the Cryonics Institute? The Cryonics Institute is the world’s leading non-profit cryonics organization bringing state of the art cryonic suspensions to the public at the most affordable price. CI was founded by the “father of cryonics,” Robert C.W. Ettinger in 1976 as a means to preserve life at liquid nitrogen temperatures. It is hoped that as the future unveils newer and more sophisticated medical nanotechnology, people preserved by CI may be restored to youth and health. 1) Cryonic Preservation 7) Funding Programs Membership qualifies you to arrange and fund a vitrification Cryopreservation with CI can be funded through approved (anti-crystallization) perfusion and cooling upon legal death, life insurance policies issued in the USA or other countries. -

PINTS for PRESS Business Park in Scottsdale, Arizona, Offers Two Types of “Cryonic Suspension” Services: Full-Body for $200,000, and APRIL 24 Head-Only for $80,000
COVER STORY THEFROZEN FIGHT FOR THE ONE SON’S MISSION TO MAKE HIS DAD WHOLE AGAIN BY TYLER HAYDEN Dr. Laurence Pilgeram didn’t believe in heaven, but HEAD he did believe in life after death. In 1990, at the age of 66, Pilgeram signed a contract with the Alcor Life Extension Foun- dation to freeze his body upon his death with the hope that, decades or centuries from now, medical science would resurrect him. Alcor, headquartered in a sand-colored PINTS FOR PRESS business park in Scottsdale, Arizona, offers two types of “cryonic suspension” services: full-body for $200,000, and APRIL 24 head-only for $80,000. It’s a bargain for Dr. Laurence Pilgeram a shot at immortality. Clients typically pay by signing over their life insurance policies. The head-only option, the company This article’s author, Tyler Hayden, will explains, is the most cost-effective way When Kurt demanded to to preserve a patient’s identity; using know why his father’s whole body COURTESY discuss the reporting and writing of this story future nanotechnology, a new hadn’t been preserved, he received conflicting with editor Matt Kettmann on Wednesday, body might be grown around accounts from Alcor, according to court records. First, the the brain. But Pilgeram never company said Laurence’s body had decayed beyond saving. Then, it April 24, 5:30 p.m., at Night Lizard liked the idea of “Neurocryo- claimed he hadn’t kept up with his yearly $525 membership dues. Finally, preservation,” his family has it suggested the technicians didn’t want to wait for the permit necessary Brewing Company in the Santa Barbara said, so he chose “Whole-Body to transport a full body across state lines. -

“Is Cryonics an Ethical Means of Life Extension?” Rebekah Cron University of Exeter 2014
1 “Is Cryonics an Ethical Means of Life Extension?” Rebekah Cron University of Exeter 2014 2 “We all know we must die. But that, say the immortalists, is no longer true… Science has progressed so far that we are morally bound to seek solutions, just as we would be morally bound to prevent a real tsunami if we knew how” - Bryan Appleyard 1 “The moral argument for cryonics is that it's wrong to discontinue care of an unconscious person when they can still be rescued. This is why people who fall unconscious are taken to hospital by ambulance, why they will be maintained for weeks in intensive care if necessary, and why they will still be cared for even if they don't fully awaken after that. It is a moral imperative to care for unconscious people as long as there remains reasonable hope for recovery.” - ALCOR 2 “How many cryonicists does it take to screw in a light bulb? …None – they just sit in the dark and wait for the technology to improve” 3 - Sterling Blake 1 Appleyard 2008. Page 22-23 2 Alcor.org: ‘Frequently Asked Questions’ 2014 3 Blake 1996. Page 72 3 Introduction Biologists have known for some time that certain organisms can survive for sustained time periods in what is essentially a death"like state. The North American Wood Frog, for example, shuts down its entire body system in winter; its heart stops beating and its whole body is frozen, until summer returns; at which point it thaws and ‘comes back to life’ 4. -

N E W S L E T T
PUBLISHED BY THE CRYONICS INSTITUTE ISSUE 03 | 2020 Cryonics insights and information for members and friends of the Cryonics Institute NEWSLETTER cryonics.org • [email protected] • 1 (866) 288-2796 CI BULLETIN I am proud to be a part of this history, and happy to report the Cryonics Institute continues to expand. Most notably, we are completing the improvements on our second facility. We are currently reviewing and finalizing plans to retrofit the facility with a bulk LN2 tank and insulated supply lines for the cryostats that will be used to store patients once the existing facility reaches capacity. On the financial front, the CI Board of Directors continues to monitor investments and operations to ensure the long term solvency of our organization. Despite challenges related to the covid epidemic, operations and patient care remain out- standing and have not slipped in the least. However, there are still some poor outcome situations that result directly from patient next of kin who are hostile to cryonics. Hello Everyone, In numerous issues of this magazine as well as in articles on our web site and our other social media venues we continue I hope you are all doing well during these trying times. With to stress the critical importance of identifying, planning and Covid, world politics, and a spattering of civil unrest it can be preparing for circumstances and situations that could cause a little depressing, but cryonicists are indeed a rare breed. a person to not be suspended. Historically, the two biggest We are known for thinking outside the box and rising above factors have been when family or friends actively block a any negative consensus. -

And Transhumanism Robert Ranisch & Stefan Lorenz Sorgner Scientific and Technological Advances Have Questioned Predominant Doctrines Concerning the Human Condition
Introducing Post- and Transhumanism Robert Ranisch & Stefan Lorenz Sorgner Scientific and technological advances have questioned predominant doctrines concerning the human condition. Transhumanism and posthumanism are among the most recent and prominent manifestations of this phenomenon. Debates on trans- and posthumanism have not only gained a considerable amount of aca- demic and popular attention recently, but have also created a widespread con- ceptual confusion. This is no surprise, considering their recent dates of origin, their conceptual similarities, and their engagements with similar questions, top- ics, and motifs. Furthermore, trans- as well as posthumanism frequently question their relationship to humanism1 and reconsider what it means to be human. In this regard both movements are streaming beyond humanism. What this means, however, is far from clear and shall be subject of discussion in this volume. In order to make sense of these two approaches and to investigate their inter- relationship, a clarification of these concepts is necessary. As a first approxima- tion, transhumanism can be seen as a stance that affirms the radical transfor- mation of human’s biological capacities and social conditions by means of tech- 1 We will not be able to address the complex histories and varieties of humanism in this chapter. Yet, the following must be noted: The word “humanism” (Humanismus) was coined in 1808 by the German theologian and philosopher Friedrich I. Niethammer in the context of educational curricula, as it is derived from the Latin word humanitas. This word has a variety of meaning but has strongly been identified with the Greek word paideia (παιδεία), e.g., i.) in Cicero’s De Oratore (I, 71) the meaning of the concept hu- manitas corresponds to that of the concept paideia; ii.) in the text Noctes Acticae (XIII, 17) by the Latin author Aulus Gellius, who lived in the 2nd century, an explicit identifi- cation of paideia and humanitas can be found. -

Cryonics Magazine, Q3 1999
Asilomar Conference Center Monterey Peninsula, Northern California USA On-site Lodging and Meals Package: To Register for Conference: Includes three excellent cafeteria-style meals Includes Social on Friday evening, June 16, 2000, each day, maid service, and use of swimming after dinner Panel on Saturday evening, and pool. Prices ($70-$150/night) dictated presentations on Saturday and Sunday. The by accommodations selected. Conference will conclude with two tracks on Non-conference guest reservations accepted. Sunday afternoon, June 18. Registration information for the Conference as well as the on-site lodging and meals pack- age at Asilomar will soon be available at www.alcor.org, or an information package can be requested by calling Alcor Life Extension Foundation at 480-905-1906. 2 Cryonics • 3rd Qtr, 1999 June 17-18, 2000: Mark Your Calendars Today! The Fourth Alcor Conference on Life Extension Technologies www.alcor.org The world is changing rapidly. Only a few years ago, most people considered the cloning Preliminary of mammals to be no more than science fiction. Repeated successes in this area, List of Speakers: however, have made it a reality today. More importantly, medical technologies like cloning and the use ofembryonic Glenna Burmer, MD, PhD, stem cells to regenerate tissues, LifeSpan BioSciences promise to make it possible to reverse all the major degenerative diseases Fred Chamberlain, within our own lifetimes. Even aging BioTransport, Inc. itself is under very heavy attack K. Eric Drexler, PhD, by today’s biological and Foresight Institute medical technologies. Gregory Fahy, PhD., 21st Century Medicine The Fourth Alcor Conference on Life Extension Technologies James Hughes, PhD, is a meeting of scientists, technologists Univ. -

Nietzsche and Transhumanism: Is Artificial Enhancement A
Nietzsche and Transhumanism: is Artificial Enhancement a Nietzschean Option? Michael James Sonne Abstract This paper will look at what Nietzsche’s metamorphoses of the spirit can tell us about being human in light of artificial enhance- ment. It will argue that advancements in science including but not limited to: designer babies, immortality movements such as cry- onics, and artificial intelligence, are veiled attempts to modify the human condition. Such attempts to modify the human condition can be viewed through the last metamorphose Nietzsche speaks of in Thus Spoke Zarathustra. The metamorphose of the child who for Nietzsche is a new beginning, a game, a self-rolling wheel, a first movement, a holy yea is the driving force behind advances in artifi- cial enhancement. This paper will argue that this is so by drawing parallels between the language of actors behind the artificial en- hancement movement, and Nietzsche’s thought behind what the metamorphose of the child would mean for the human condition. The paper will argue that Nietzsche’s metamorphose of the child is a positive vision of the potential of human endeavours. The paper will conclude that artificial enhancement can be viewed through this metamorphose of spirit, insofar as its enhancements are taken to be advancements for humans; enhancement should enhance the human condition as opposed to overcoming the human condition. Keywords: Transhumanism, eternal return, dignity, metamorphose. 1 Nietzsche and Transhumanism The question of whether Nietzsche qualifies as a transhumanist is not the primary purpose of this paper. Debates as to whether Nietzsche is a transhumanist have been discussed in the literature 1, resulting in different conclusions. -

N E W S L E T T
PUBLISHED BY THE CRYONICS INSTITUTE ISSUE 02 | 2021 Cryonics insights and information for members and friends of the Cryonics Institute NEWSLETTER cryonics.org • [email protected] • 1 (866) 288-2796 CI BULLETIN online Zoom meeting option for those who can’t attend in person or are hesitant to travel due to covid concerns. The meeting is open to the public, so it is also a great opportunity for prospective members to meet fellow cryonicists and learn more about the Cryonics Institute. If you subscribe to our magazine but are not a member, let me personally invite you to attend this year’s meeting either in person or online to get to know CI, our Team and our Members. The year’s AGM will be held Sunday, September12th. There will be tours of the CI main facility as well as “CI West,” our new additional facility location prior to the main meeting. Full details can be found on page 8, as well as on cryonics.org. Hello all, Facility Expansion Structural renovations have been completed on the new facil- It’s that time of year again! We’re coming up on Ci’s Annual ity, and late this year or early next year we will be installing the General Meeting & elections where members will be elect- bulk liquid nitrogen tank, LN2 delivery lines and begin adding ing four candidates for the Board of Directors. Currently we cryostats ready to start storing additional patients as needed. have four incumbents and three new challengers who have It is in our business model to be ready and able to expand as tossed their hats in the ring. -
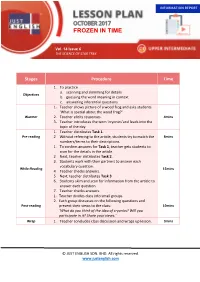
Frozen in Time Upper Intermediate FROZEN in TIME
INFORMATION REPORT LESSON PLAN Just English Magazine Vol. 14 Issue 6 – THE SCIENCE OF STAR TREK OCTOBER 2017 Frozen in Time Upper Intermediate FROZEN IN TIME Vol. 14 Issue 6 THE SCIENCE OF STAR TREK Stages Procedure Time 1. To practice a. scanning and skimming for details Objectives b. guessing the word meaning in context c. answering inferential questions 1. Teacher shows picture of a wood frog and asks students ‘What is special about the wood frog?’ Warmer 2. Teacher elicits responses. 4mins 3. Teacher introduces the term ‘cryonics’and leads into the topic of the day. 1. Teacher distributes Task 1. Pre‐reading 2. Without referring to the article, students try to match the 8mins numbers/terms to their descriptions. 1. To confirm answers for Task 1, teacher gets students to scan for the details in the article. 2. Next, teacher distributes Task 2. 3. Students work with their partners to answer each vocabulary question. While‐Reading 15mins 4. Teacher checks answers. 5. Next, teacher distributes Task 3. 6. Students skim and scan for information from the article to answer each question. 7. Teacher checks answers. 1. Teacher divides class into small groups. 2. Each group discusses on the following questions and Post‐reading present their views to the class: 10mins ‘What do you think of the idea of cryonics? Will you participate in it? Share your views.’ Wrap 1. Teacher concludes class discussion and wraps up lesson. 3mins © JUST ENGLISH SDN. BHD. All rights reserved. www.justenglish.com LESSON PLAN Just English Magazine Vol. 14 Issue 6 – THE SCIENCE OF STAR TREK Planting for the Future Upper Intermediate TASK 1 Match each number or term below to its description. -

The Prospect of Immortality
Robert C. W. Ettinger__________The Prospect Of Immortality Contents Preface by Jean Rostand Preface by Gerald J. Gruman Foreword Chapter 1. Frozen Death, Frozen Sleep, and Some Consequences Suspended Life and Suspended Death Future and Present Options After a Moment of Sleep Problems and Side Effects Chapter II. The Effects of Freezing and Cooling Long-term Storage Successes in Freezing Animals and Tissues The Mechanism of Freezing Damage Frostbite The Action of Protective Agents The Persistence of Memory after Freezing The Extent of Freezing Damage Rapid Freezing and Perfusion Possibilities The Limits of Delay in Treatment The Limits of Delay in Cooling and Freezing Maximum and Optimum Storage Temperature Radiation Hazard Page 1 Robert Ettinger – All Rights Reserved www.cryonics.org Robert C. W. Ettinger__________The Prospect Of Immortality Chapter III. Repair and Rejuvenation Revival after Clinical Death Mechanical Aids and Prostheses Transplants Organ Culture and Regeneration Curing Old Age Chapter IV. Today's Choices The Outer Limits of Optimism Preserving Samples of Ourselves Preserving the Information Organization and Organizations Emergency and Austerity Freezing Freezing with Medical Cooperation Individual Responsibility: Dying Children Husbands and Wives, Aged Parents and Grandparents Chapter V. Freezers and Religion Revival of the Dead: Not a New Problem The Question of God's Intentions The Riddle of Soul Suicide Is a Sin God's Image and Religious Adaptability Added Time for Growth and Redemption Conflict with Revelation The Threat of Materialism Perspective Chapter VI. Freezers and the Law Freezers and Public Decency Definitions of Death; Rights and Obligations of the Frozen Life Insurance and Suicide Mercy Killings Murder Widows, Widowers, and Multiple Marriages Cadavers as Citizens Potter's Freezer and Umbrellas Page 2 Robert Ettinger – All Rights Reserved www.cryonics.org Robert C. -

The Hostile Spouse Is Almost Exclusively a Female Phenomenon
A Literal Death Sentence One unpleasant issue in cryonics is the "hostile wife" phenomenon. The authors of this article know of a number of high profile cryonicists who need to hide their cryonics activities from their wives and ex-high profile cryonicists who had to choose between cryonics and their relationship. We also know of men who would like to make cryonics arrangements but have not been able to do so because of resistance from their wives or girlfriends. In such cases, the female partner can be described as nothing less than hostile toward cryonics. As a result, these men face certain death as a consequence of their partner's hostility. While it is not unusual for any two people to have differing points of view regarding cryonics, men are more interested in making cryonics arrangements. A recent membership update from the Alcor Life Extension Foundation reports that 667 males and 198 females have made cryonics arrangements. Although no formal data are available, it is common knowledge that a substantial number of these female cryonicists signed up after being persuaded by their husbands or boyfriends. For whatever reason, males are more interested in cryonics than females. These issues raise an obvious question: are women more hostile to cryonics than men? There is no direct answer to this question since the requisite data have not been collected. However, both the gravity and magnitude of the problem, as we are about to detail, suggests this as a fertile, if not urgent, area for future research. One consequence of men being more interested in cryonics than women is that heterosexual men are more often faced with hostile wives and girlfriends than the other way round. -

Curtis Henderson Page 3
3rd Quarter 2009 • Volume 30:3 Cover Story Impressions of Curtis Henderson page 3 Curtis Henderson: Cryonics Pioneer Member Profile: John Schloendorn page 12 page 16 Curtis Henderson [1926 - 2009 - ...] ISSN 1054-4305 $9.95 Improve Your Odds of a Good Cryopreservation You have your cryonics funding and contracts in place but have you considered other steps you can take to prevent problems down the road? þ Do you keep Alcor up-to-date about personal and medical changes? þ Does your Alcor paperwork still reflect your current wishes? þ Have you executed a cryonics-friendly Living Will and Durable Power of Attorney for Health Care? þ Do you wear your bracelet and talk to your friends and family about your desire to be cryopreserved? þ Do you have hostile relatives or supportive relatives that are willing to sign a Relative’s Affidavit? þ Do you attend local cryonics meetings or are you interested in starting a local group yourself? þ Are you interested in contributing to Alcor? Contact Alcor at 1-877-462-5267 and let us know how we can assist you. Take a look at the Alcor Blog www.alcornews.org/weblog Your source for news about: Cryonics technology Cryopreservation cases Television programs about cryonics Speaking events and meetings Employment opportunities 3RD QUARTER 2009 • V OLUME 30:3 e 30:3 Volum 2009 • arter 3rd Qu y Stor over s Contents C ssion mpre I rtis f Cu n o erso Hend : ofile age 3 r Pr p mbe dorn Me loen Sch e 16 rtis : John CpagOVER STORY: PAGE 3 Cu rson nde eer He Pion 16 Member Profile: nics Cryo 12 page Alcor staff member and John Schloendorn icsryonics historian Mike Meet John Schloendorn, Curt rPseorrny remembers Curtis ende Alcor member and H - ...] 200H9 enderson and his [1926 - upcoming young anti- important role in the aging researcher.