Left Pulmonary Artery from the Ascending Aorta: a Case Report and Review of Published Cases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Of the Pediatric Mediastinum
MRI of the Pediatric Mediastinum Dianna M. E. Bardo, MD Director of Body MR & Co-Director of the 3D Innovation Lab Disclosures Consultant & Speakers Bureau – honoraria Koninklijke Philips Healthcare N V Author – royalties Thieme Publishing Springer Publishing Mediastinum - Anatomy Superior Mediastinum thoracic inlet to thoracic plane thoracic plane to diaphragm Inferior Mediastinum lateral – pleural surface anterior – sternum posterior – vertebral bodies Mediastinum - Anatomy Anterior T4 Mediastinum pericardium to sternum Middle Mediastinum pericardial sac Posterior Mediastinum vertebral bodies to pericardium lateral – pleural surface superior – thoracic inlet inferior - diaphragm Mediastinum – MR Challenges Motion Cardiac ECG – gating/triggering Breathing Respiratory navigation Artifacts Intubation – LMA Surgical / Interventional materials Mediastinum – MR Sequences ECG gated/triggered sequences SSFP – black blood SE – IR – GRE Non- ECG gated/triggered sequences mDIXON (W, F, IP, OP), eTHRIVE, turbo SE, STIR, DWI Respiratory – triggered, radially acquired T2W MultiVane, BLADE, PROPELLER Mediastinum – MR Sequences MRA / MRV REACT – non Gd enhanced Gd enhanced sequences THRIVE, mDIXON, mDIXON XD Mediastinum – Contents Superior Mediastinum PVT Left BATTLE: Phrenic nerve Vagus nerve Structures at the level of the sternal angle Thoracic duct Left recurrent laryngeal nerve (not the right) CLAPTRAP Brachiocephalic veins Cardiac plexus Aortic arch (and its 3 branches) Ligamentum arteriosum Thymus Aortic arch (inner concavity) Trachea Pulmonary -

Thoracic Aorta
GUIDELINES AND STANDARDS Multimodality Imaging of Diseases of the Thoracic Aorta in Adults: From the American Society of Echocardiography and the European Association of Cardiovascular Imaging Endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance Steven A. Goldstein, MD, Co-Chair, Arturo Evangelista, MD, FESC, Co-Chair, Suhny Abbara, MD, Andrew Arai, MD, Federico M. Asch, MD, FASE, Luigi P. Badano, MD, PhD, FESC, Michael A. Bolen, MD, Heidi M. Connolly, MD, Hug Cuellar-Calabria, MD, Martin Czerny, MD, Richard B. Devereux, MD, Raimund A. Erbel, MD, FASE, FESC, Rossella Fattori, MD, Eric M. Isselbacher, MD, Joseph M. Lindsay, MD, Marti McCulloch, MBA, RDCS, FASE, Hector I. Michelena, MD, FASE, Christoph A. Nienaber, MD, FESC, Jae K. Oh, MD, FASE, Mauro Pepi, MD, FESC, Allen J. Taylor, MD, Jonathan W. Weinsaft, MD, Jose Luis Zamorano, MD, FESC, FASE, Contributing Editors: Harry Dietz, MD, Kim Eagle, MD, John Elefteriades, MD, Guillaume Jondeau, MD, PhD, FESC, Herve Rousseau, MD, PhD, and Marc Schepens, MD, Washington, District of Columbia; Barcelona and Madrid, Spain; Dallas and Houston, Texas; Bethesda and Baltimore, Maryland; Padua, Pesaro, and Milan, Italy; Cleveland, Ohio; Rochester, Minnesota; Zurich, Switzerland; New York, New York; Essen and Rostock, Germany; Boston, Massachusetts; Ann Arbor, Michigan; New Haven, Connecticut; Paris and Toulouse, France; and Brugge, Belgium (J Am Soc Echocardiogr 2015;28:119-82.) TABLE OF CONTENTS Preamble 121 B. How to Measure the Aorta 124 I. Anatomy and Physiology of the Aorta 121 1. Interface, Definitions, and Timing of Aortic Measure- A. The Normal Aorta and Reference Values 121 ments 124 1. -

Surgical Indications. a Critical Point of View
Surgical indications in ascending aorta aneurysms: What do we know? Jean-Luc MONIN, MD, PhD. Institut Mutualiste Montsouris, Paris, FRANCE Disclosures related to this talk : www.imm.fr NONE 2 Clinical case www.imm.fr • A 40 year-old woman • Height: 172 cm/ Weight: 70 kg • Ascending aortic aneurysm • She wants to become pregnant (3-year old kid) • No Marfan syndrome or bicuspid AV • Younger sister: sudden unexplained death (at 18 years old) • 2012 (MRI): Valsalva: 42 mm, tubular: 43 mm • 2013 (MRI): Valsalva: 40 mm, tubular: 45 mm 3 www.imm.fr www.imm.fr www.imm.fr www.imm.fr www.imm.fr www.imm.fr What would we advise to this woman www.imm.fr ? A. Pregnancy is temporarily contra indicated, MRI or cardiac CT is needed B. No contra indication for pregnancy, TTE or MRI at 1 year 10 Thoracic aortic aneurysm www.imm.fr Thoracic Aortic Aneurysm: An indolent but virulent process www.imm.fr Ascending Aorta 1 mm/ year 45 mm 60 mm 3 mm/ year 34% Rate of aortic dilatation is EXPONENTIAL • Expansion rate ≈ 2.1 mm/ year for an initial diameter of 35-40 mm (%) rupture dissection/ of risk Lifetime Aortic diameter (cm) • Expansion rate ≈ 5.6 mm/ year for aneurysms ≥ 60 mm Coady et al. J Thorac Cardiovasc Surg. 1997;113: 476 Mechanical properties of human ascending aorta : >6 cm is the limit www.imm.fr Exponential relationship between wall stress and aneurysm size in ascending aortic aneurysms. • Pink columns : SBP =100 mm Hg • Purple columns : SBP = 200 mm Hg • Range of maximum tensile strength of the human aorta : 800 to 1,000 kPa Koullias et al. -

Aorta and the Vasculature of the Thorax
Aorta and the Vasculature of the Thorax Ali Fırat Esmer, MD Ankara University Faculty of Medicine Department of Anatomy THE AORTA After originating from left ventricle, it ascends for a short distance, arches backward and to the left side, descends within the thorax on the left side of the vertebral column It is divided for purposes of The aorta is the main arterial trunk description into: that delivers oxygenated blood from Ascending aorta the left ventricle of the heart to the Arch of the aorta and tissues of the body. Descending aorta (thoracic and abdominal aorta) Ascending Aorta The ascending aorta begins at the base of the left ventricle runs upward and forward at the level of the sternal angle, where it becomes continuous with the arch of the aorta it possesses three bulges, the sinuses of the aorta Branches Right coronary artery Left coronary artery ARCH OF THE AORTA The aortic arch is a continuation of the ascending aorta and begins at the level of the second sternocostal joint. • It arches superiorly, posteriorly and to the left before moving inferiorly. • The aortic arch ends at the level of the T4 vertebra / at level of sternal angle. Branches; Brachiocephalic artery (Innominate artery) Left common carotid artery Left subclavian artery It begins when the ascending aorta emerges from the pericardial sac and courses upward, backward, and to the left as it passes through the superior mediastinum, ending on the left side at vertebral level TIV/V. Extending as high as the midlevel of the manubrium of the sternum, the arch is initially anterior and finally lateral to the trachea. -

Aneurysm of the Ascending Aorta Presenting with Pulmonary Stenosis
Thorax: first published as 10.1136/thx.21.3.236 on 1 May 1966. Downloaded from Thorax (1966), 21, 236. Aneurysm of the ascending aorta presenting with pulmonary stenosis M. H. YACOUB, M. V. BRAIMBRIDGE,1 AND R. G. GOLD From the Brornpton Hospital, London S.W.3 The symptoms and signs of aneurysms of the The chest radiograph (Fig. 1) showed old bilateral thoracic aorta are due mainly to compression of apical tuberculosis, and both lungs were emphyse- surrounding mediastinal structures and depend on matous. The emphysema was most marked in the left the size and location of the aneurysm. Compres- upper lobe with bullae present. A hemispherical mass, sion of the trachea, bronchi, oesophagus, recurrent 9 by 6 cm., lay to the left and in front of the ascend- ing aorta. Its border was slightly irregular. and laryngeal nerve, bone, and superior vena cava is showed flecks of linear calcification laterally and common, but pressure on the pulmonary artery has superiorly. The heart size was within normal limits. not been described in spite of its intimate relation At right heart catheterization difficulty was experi- to the ascending aorta. enced in passing the catheter into the pulmonary The object of this paper is to describe a case artery. The right ventricular systolic pressure was presenting in this way and to discuss the particular 44 mm. Hg and the pulmonary artery systolic pres- surgical problems involved in the excision of such sure was 18 mm. Hg. the systolic gradient being 24 an aneurysm. mm. Hg, with a single change in pressure fromarterial to ventricular configuration in the vicinity of the pulmonary valve. -

Aortic Disease
Aortic Disease The Aorta Center in Cleveland Clinic’s Heart & Vascular Institute is organized to optimize the Cleveland Clinic’s Acute care of patients and to facilitate collaboration across disciplines with a focus on conditions that Aortic Treatment affect all segments of the aorta. This multidisciplinary effort has resulted in the busiest aorta center in the US, leading the way in quality, innovation, and research. Center provides rapid transport, treatment, and follow-up for patients with aortic dissection Aortic Surgery and impending aneurysm Volume and Type rupture. In 2016, 5634 2012 – 2016 patients were transported by Cleveland Clinic’s Critical Volume 2016 Totals Care Transport team. More 1500 Open ascending/ than 20% of the patients arch repair (N = 709) transported were treated in Open descending/ the Sydell and Arnold Miller thoracoabdominal repair (N = 34) 1000 Family Heart & Vascular Endovascular descending/ Institute, and many had thoracoabdominal repair (N = 265) acute aortic syndromes. 500 Open abdominal repair (N = 102) Call 877.379.CODE (2633) Endovascular abdominal to expedite the transfer of repair (N = 90) 0 patients with acute aortic 2012 2013 2014 2015 2016 Endovascular ascending aorta syndromes. N = 1194 1219 1230 1185 1228 repair (N = 28) In-Hospital Mortality (N = 1228) 2016 Percent 30 Elective Expected Emergency 20 10 0 0 Ascending/Arch Descending TAAA AAA Open/Endovascular AAA = abdominal aortic aneurysm, TAAA = thoracoabdominal aortic aneurysm Source: Data from the Vizient Clinical Data Base/Resource ManagerTM used by permission of Vizient. All rights reserved. Sydell and Arnold Miller Family Heart & Vascular Institute 41 Aortic Disease Redefining When to Operate Ascending Aorta and Aortic Arch Open Surgery Volume 2012 – 2016 Volume 800 600 400 200 0 2012 2013 2014 2015 2016 N =6728 76 762720 709 In 2016, Cleveland Clinic surgeons performed 709 open procedures to repair the ascending aorta and aortic arch. -

Pericardial Tamponade Caused by a Perforating Atherosclerotic Ulcer of the Ascending Aorta
Netherlands Journal of Critical Care Copyright © 2010, Nederlandse Vereniging voor Intensive Care. All Rights Reserved. Received May 2010; accepted August 2010 CASEREPORT Pericardialtamponadecausedbyaperforating atheroscleroticulceroftheascendingaorta EMvanDriel1,PKlein2,EdeJonge1,MSArbous1 1DepartmentofIntensiveCare,LeidenUniversityMedicalCenter,Leiden,TheNetherlands 2DepartmentofCardio-Thoracicsurgery,LeidenUniversityMedicalCenter,Leiden,TheNetherlands Abstract-We present the case of a patient with pericardial tamponade caused by a rare complication of atherosclerosis. An eighty- year-old male suffered a witnessed cardiac arrest due to pulseless electrical activity (PEA). Transthoracic echocardiography showed a pericardial effusion of 1.5 to 2 cm. Emergency subcostal pericardiocentesis drained approximately 15 ml of sanquinolent fluid. sub- sequent CT scan of the thorax showed a penetrating atherosclerotic ulcer (PAU) of the proximal ascending aorta with signs of active bleeding into the pericardium and right pleural cavity. The patient was diagnosed with a pericardial tamponade and haematothorax due to a perforated PAU of the ascending aorta, resulting in PEA. Although a PAU of the descending aorta and distal aortic arch can be treated by endovascular repair (EVAR), open thoracic surgery remains the treatment of choice for lesions located in the ascending aorta or proximal mid-aortic arch. Keywords- Cardiac tamponade, penetrating atherosclerotic ulcer, CT scan, endovascular repair, surgical repair, atherosclerotic disease. Introduction -

Superior and Posterior Mediastina Reading: 1. Gray's Anatomy For
Dr. Weyrich G07: Superior and Posterior Mediastina Reading: 1. Gray’s Anatomy for Students, chapter 3 Objectives: 1. Subdivisions of mediastinum 2. Structures in Superior mediastinum 3. Structures in Posterior mediastinum Clinical Correlate: 1. Aortic aneurysms Superior Mediastinum (pp.181-199) 27 Review of the Subdivisions of the Mediastinum Superior mediastinum Comprises area within superior thoracic aperture and transverse thoracic plane -Transverse thoracic plane – arbitrary line from the sternal angle anteriorly to the IV disk or T4 and T5 posteriorly Inferior mediastinum Extends from transverse thoracic plane to diaphragm; 3 subdivisions Anterior mediastinum – smallest subdivision of mediastinum -Lies between the body of sternum and transversus thoracis muscles anteriorly and the pericardium posteriorly -Continuous with superior mediastinum at the sternal angle and limited inferiorly by the diaphragm -Consists of sternopericardial ligaments, fat, lymphatic vessels, and branches of internal thoracic vessels. Contains inferior part of thymus in children Middle mediastinum – contains heart Posterior mediastinum Superior Mediastinum Thymus – lies posterior to manubrium and extends into the anterior mediastinum -Important in development of immune system through puberty -Replaced by adipose tissue in adult Arterial blood supply -Anterior intercostals and mediastinal branches of internal thoracic artery Venous blood supply -Veins drain into left brachiocephalic, internal thoracic, and thymic veins 28 Brachiocephalic Veins - Formed by the -
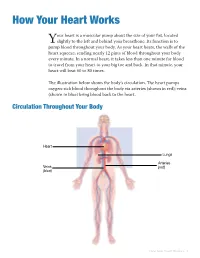
How Your Heart Works
How Your Heart Works our heart is a muscular pump about the size of your fist, located slightly to the left and behind your breastbone. Its function is to Ypump blood throughout your body. As your heart beats, the walls of the heart squeeze, sending nearly 12 pints of blood throughout your body every minute. In a normal heart, it takes less than one minute for blood to travel from your heart to your big toe and back. In that minute, your heart will beat 60 to 80 times. The illustration below shows the body’s circulation. The heart pumps oxygen-rich blood throughout the body via arteries (shown in red); veins (shown in blue) bring blood back to the heart. Circulation Throughout Your Body Heart Lungs Arteries Veins (red) (blue) How Your Heart Works • 1 Heart Anatomy The heart has two sides, separated by an inner wall called theseptum . The right side of the heart pumps blood to the lungs to pick up oxygen. The left side of the heart receives the oxygen-rich blood from the lungs and pumps it to the body. The heart has four chambers and four valves and is connected to various blood vessels. Veins are blood vessels that carry blood from the body to the heart. Arteries are blood vessels that carry blood away from the heart to the body. The illustration shows a cross-section of a healthy heart with its inside structures. The explanations of these structures are listed on the next page. Aorta (to body) Superior vena cava (from upper body) Pulmonary artery Left pulmonary arteries Right pulmonary (to left lung) arteries (to right lung) Left pulmonary Right pulmonary veins (from left lung) veins (from right Left Heart: Right Heart: Left atrium Right atrium Aortic valve Pulmonary valve Mitral valve Tricuspid valve Left ventricle Right ventricle Septum Inferior vena cava (from lower body) Aorta 2 • Heart Surgery: A Guide for Patients and Their Families • Michigan Medicine Heart Chambers The heart has four chambers. -

Approach to Mediastinal Masses with Focus on Felson’S Classification and Anatomical Basis of Lines and Stripes in the Chest
Dr. Pratik Mukherjee, MMed, FRCR Dr. Ashish Chawla, MD, ABR (USA) Khoo Teck Puat Hospital, Singapore The authors declare no financial disclosures. To revisit the basics of approach to mediastinal masses with focus on Felson’s classification and anatomical basis of lines and stripes in the chest. To achieve a higher degree of confidence in localizing mediastinal masses on conventional radiographs and follow up cross-sectional imaging. To be aware of certain pitfalls and mimics of true mediastinal masses. LOCATION ! LOCATION ! LOCATION ! Mediastinal masses occur in PREDICTABLE LOCATIONS based on tissue of origin in that compartment. Hence…. LOCATION of mass is the single most important criteria for coming to a diagnosis. Antero-superior masses are most common. Divided into 3 main compartments according to Felson’s classification: Anterior Middle Posterior Mediastinum Mediastinum Mediastinum Superior - imaginary line traversing the manubriosternal joint and the lower surface of the fourth thoracic vertebra (T4). Inferior – Anterior Middle Posterior Felson’ method - line extending from the diaphragm to the thoracic inlet along the back of the heart and anterior to the trachea separates the anterior and middle mediastinum. A line that connects points 1 cm behind the anterior margins of the vertebral bodies separates the middle and posterior mediastinal compartments. Most cases are asymptomatic and finding of mediastinal mass is incidental ! Step by step approach: 1. Posteroanterior and lateral chest radiograph - from which compartment does mass arise - appearance of mass e.g. fat, fluid, calcification - “lines and stripes” the mass displaces 2. CT (or MRI) to better characterize nature and extent of lesion. (MRI especially good at looking for spinal canal invasion in masses of posterior mediastinum.) 3. -

Aortic Valve and Ascending Aorta Guidelines for Management and Quality Measures Lars G
Aortic Valve and Ascending Aorta Guidelines for Management and Quality Measures Lars G. Svensson, David H. Adams, Robert O. Bonow, Nicholas T. Kouchoukos, D. Craig Miller, Patrick T. O'Gara, David M. Shahian, Hartzell V. Schaff, Cary W. Akins, Joseph E. Bavaria, Eugene H. Blackstone, Tirone E. David, Nimesh D. Desai, Todd M. Dewey, Richard S. D'Agostino, Thomas G. Gleason, Katherine B. Harrington, Susheel Kodali, Samir Kapadia, Martin B. Leon, Brian Lima, Bruce W. Lytle, Michael J. Mack, Michael Reardon, T. Brett Reece, G. Russell Reiss, Eric E. Roselli, Craig R. Smith, Vinod H. Thourani, E. Murat Tuzcu, John Webb and Mathew R. Williams Ann Thorac Surg 2013;95:1-66 DOI: 10.1016/j.athoracsur.2013.01.083 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://ats.ctsnetjournals.org/cgi/content/full/95/6_Supplement/S1 The Annals of Thoracic Surgery is the official journal of The Society of Thoracic Surgeons and the Southern Thoracic Surgical Association. Copyright © 2013 by The Society of Thoracic Surgeons. Print ISSN: 0003-4975; eISSN: 1552-6259. Downloaded from ats.ctsnetjournals.org by on May 28, 2013 SPECIAL REPORT Aortic Valve and Ascending Aorta Guidelines for Management and Quality Measures Writing Committee Members: Lars G. Svensson, MD, PhD (Chair), David H. Adams, MD (Vice-Chair), Robert O. Bonow, MD (Vice-Chair), Nicholas T. Kouchoukos, MD (Vice-Chair), D. Craig Miller, MD (Vice-Chair), Patrick T. O’Gara, MD (Vice-Chair), David M. Shahian, MD (Vice-Chair), Hartzell V. Schaff, MD (Vice-Chair), Cary W. -

A Rare Case of an Ascending Aorta and Aortic Arch Aneurysm with An
ISSN: 2643-4571 Olalo and Guillermo. Int J Rare Dis Disord 2020, 3:026 DOI: 10.23937/2643-4571.1710026 Volume 3 | Issue 2 International Journal of Open Access Rare Diseases & Disorders CASE REPORT A Rare Case of an Ascending Aorta and Aortic Arch Aneurysm with an Aberrant Right Common Carotid Artery and a Proximal Descending Aortic Ectasia Mikhail M Olalo, MD* and Syril Bren P Guillermo, MD, FPCP, FPCC Check for updates Department of Internal Medicine, Southern Philippines Medical Center, Philippines *Corresponding author: Mikhail M Olalo, MD, Department of Internal Medicine, Southern Philippines Medical Center, Philippines Abstract Objectives Ascending aortic aneurysms are asymptomatic and are This case report aims to: usually discovered as an incidental finding on chest imag- ing. However, larger aneurysms can present with symptoms 1. Present a case of a 63-year-old male complaining of resulting from compression of surrounding structures in- sudden onset of severe dull back pain; cluding the trachea, bronchi, and the esophagus which can result in hoarseness, cough chest pain or back pain. 2. Discuss the approach and management of the case; and The presence of an aortic arch anomaly, specifically an aberrant right common carotid artery, in a background of 3. Present the incidence and prognosis of the case. an aortic arch aneurysm is extremely rare with a worldwide incidence of < 1%. They are usually asymptomatic but can Introduction result to catastrophic life-threatening events and pose sig- nificant challenges to surgical or endovascular treatment. Ascending aortic aneurysm is the dilatation of the aortic vessel to more than 150% of the diameter expect- This is a case of a 63-year-old Filipino male who presented with a sudden onset of dull back pain radiating to the left an- ed for sex, age, and body weight while lesser dilatations terior chest.