12(2) 06 Ivanova.Indd
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annex REPORT for 2019 UNDER the “HEALTH CARE” PRIORITY of the NATIONAL ROMA INTEGRATION STRATEGY of the REPUBLIC of BULGAR
Annex REPORT FOR 2019 UNDER THE “HEALTH CARE” PRIORITY of the NATIONAL ROMA INTEGRATION STRATEGY OF THE REPUBLIC OF BULGARIA 2012 - 2020 Operational objective: A national monitoring progress report has been prepared for implementation of Measure 1.1.2. “Performing obstetric and gynaecological examinations with mobile offices in settlements with compact Roma population”. During the period 01.07—20.11.2019, a total of 2,261 prophylactic medical examinations were carried out with the four mobile gynaecological offices to uninsured persons of Roma origin and to persons with difficult access to medical facilities, as 951 women were diagnosed with diseases. The implementation of the activity for each Regional Health Inspectorate is in accordance with an order of the Minister of Health to carry out not less than 500 examinations with each mobile gynaecological office. Financial resources of BGN 12,500 were allocated for each mobile unit, totalling BGN 50,000 for the four units. During the reporting period, the mobile gynecological offices were divided into four areas: Varna (the city of Varna, the village of Kamenar, the town of Ignatievo, the village of Staro Oryahovo, the village of Sindel, the village of Dubravino, the town of Provadia, the town of Devnya, the town of Suvorovo, the village of Chernevo, the town of Valchi Dol); Silistra (Tutrakan Municipality– the town of Tutrakan, the village of Tsar Samuel, the village of Nova Cherna, the village of Staro Selo, the village of Belitsa, the village of Preslavtsi, the village of Tarnovtsi, -

1 I. ANNEXES 1 Annex 6. Map and List of Rural Municipalities in Bulgaria
I. ANNEXES 1 Annex 6. Map and list of rural municipalities in Bulgaria (according to statistical definition). 1 List of rural municipalities in Bulgaria District District District District District District /Municipality /Municipality /Municipality /Municipality /Municipality /Municipality Blagoevgrad Vidin Lovech Plovdiv Smolyan Targovishte Bansko Belogradchik Apriltsi Brezovo Banite Antonovo Belitsa Boynitsa Letnitsa Kaloyanovo Borino Omurtag Gotse Delchev Bregovo Lukovit Karlovo Devin Opaka Garmen Gramada Teteven Krichim Dospat Popovo Kresna Dimovo Troyan Kuklen Zlatograd Haskovo Petrich Kula Ugarchin Laki Madan Ivaylovgrad Razlog Makresh Yablanitsa Maritsa Nedelino Lyubimets Sandanski Novo Selo Montana Perushtitsa Rudozem Madzharovo Satovcha Ruzhintsi Berkovitsa Parvomay Chepelare Mineralni bani Simitli Chuprene Boychinovtsi Rakovski Sofia - district Svilengrad Strumyani Vratsa Brusartsi Rodopi Anton Simeonovgrad Hadzhidimovo Borovan Varshets Sadovo Bozhurishte Stambolovo Yakoruda Byala Slatina Valchedram Sopot Botevgrad Topolovgrad Burgas Knezha Georgi Damyanovo Stamboliyski Godech Harmanli Aitos Kozloduy Lom Saedinenie Gorna Malina Shumen Kameno Krivodol Medkovets Hisarya Dolna banya Veliki Preslav Karnobat Mezdra Chiprovtsi Razgrad Dragoman Venets Malko Tarnovo Mizia Yakimovo Zavet Elin Pelin Varbitsa Nesebar Oryahovo Pazardzhik Isperih Etropole Kaolinovo Pomorie Roman Batak Kubrat Zlatitsa Kaspichan Primorsko Hayredin Belovo Loznitsa Ihtiman Nikola Kozlevo Ruen Gabrovo Bratsigovo Samuil Koprivshtitsa Novi Pazar Sozopol Dryanovo -

Rila Monastery Nature Park Management Plan 2004-2013
The Minister of the Environment and Waters D. Arsenova Rila Monastery Nature Park Management Plan 2004-2013 DRAFT Adopted by Decision # ххх of the Council of Ministers dated хх.хх, 2004 Presented by ARD/BCEGP in fulfillment of Terms of Reference commissioned by the Ministry of the Environment and Waters, # хх-хх-хххх, March 2001 The drafting and publication of this Management Plan was made possible through the generous support of the Environment, Energy and Social Transition Department of the Europe and Eurasia Desk of the United States Agency for International Development, pursuant to Contract # LAG-I-00-99-00013-00. All opinions expressed herein are solely at the authors’ discretion and do not necessarily reflect the position of the United States Agency for International Development. February, 2004 Team of Authors The Core Planning Team which drafted the present Management Plan for Rila Monastery Nature Park comprises the following members: Dr. Petar Yankov D.Sc. (ecology/zoology), Dr. Dimitar Peev D.Sc. (ecology/botany), Eng. Ventsisval Velichkov (forest engineer), Mrs. Snezhana Kostadinova (sociologist), as well as the members of the Coordinating Team of the BCEG Project, as follows: Dr. Peter Hetz (team leader), Dimitrina Boteva, MSc. (biodiversity expert) and Gergana Pavlova (administrative support). The Extended Planning Team comprises the following members: Eng. Mihail Mihailov, Director of Rila Monastery Nature Park; Ms. Nikolina Georgieva, biodiversity expert with the Directorate Rila Monastery NP, Eng. Vassil Petrov, acting Director of Rila NP, His Eminence Gabriel, Metropolitan Bishop of Lovech, representative of the Holy Synod of the Bulgarian Orthodox Church; the Most Reverend Bishop John, Abbot of Rila Monastery, Eng. -

New and Unpublished Data About Bulgarian Ground Beetles from the Tribes Pterostichini, Sphodrini, and Platynini (Coleoptera, Carabidae)
Acta Biologica Sibirica 7: 125–141 (2021) doi: 10.3897/abs.7.e67015 https://abs.pensoft.net RESEARCH ARTICLE New and unpublished data about Bulgarian ground beetles from the tribes Pterostichini, Sphodrini, and Platynini (Coleoptera, Carabidae) Teodora Teofilova1 1 Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences, 1 Tsar Osvoboditel Blvd., 1000, Sofia, Bulgaria. Corresponding author: Teodora Teofilova ([email protected]) Academic editor: R. Yakovlev | Received 6 April 2021 | Accepted 22 April 2021 | Published 20 May 2021 http://zoobank.org/53E9E1F4-2338-494C-870D-F3DA4AA4360B Citation: Teofilova T (2021) New and unpublished data about Bulgarian ground beetles from the tribes Pterostichini, Sphodrini, and Platynini (Coleoptera, Carabidae). Acta Biologica Sibirica 7: 125–141. https://doi. org/10.3897/abs.7.e67015 Abstract Bulgarian ground beetle (Coleoptera, Carabidae) fauna is relatively well studied but there are still many species and regions in the country which are not well researched. The present study aims at complementing the data about the distribution of the carabids from the tribes Pterostichini, Spho- drini, and Platynini, containing many diverse, interesting, and endemic species. It gives new records for 67 species and 23 zoogeographical regions in Bulgaria. The material was collected in the period from 1926 to 2021 through different sampling methods. Twenty-three species are recorded for the first time in different regions. Six species are reported for the second time in the regions where they were currently collected. Thirty-one species have not been reported for more than 20 years in Eastern and Middle Stara Planina Mts., Kraishte region, Boboshevo-Simitli valley, Sandanski-Petrich valley, Lyulin Mts., Vitosha Mts., Rila Mts., Pirin Mts., Slavyanka Mts., Thracian Lowland, and Sakar-Tundzha re- gion. -

Blagovest Sendov February 8, 1932-January 19
Available online at www.sciencedirect.com ScienceDirect Journal of Approximation Theory 254 (2020) 105406 www.elsevier.com/locate/jat In Memoriam: Blagovest Sendov February 8, 1932–January 19, 2020 Blagovest Hristov Sendov was born on February 8, 1932 in Asenovgrad, Bulgaria. From a very early age, Sendov showed an exceptional talent for mathematics. His strong desire to study mathematics at the Sofia University however could not be realized immediately. Being from a bourgeois family he was not allowed to study at the University. The communist system in Bulgaria at that time did not let young people of unreliable bourgeois background into higher education. With his typical perseverance and creativity he managed to overcome this first serious obstacle in his life. After being a laborer for three years, cleaning the streets in Sofia, he was admitted to Sofia University. Bl. Sendov graduated from the Department of Mathematics at Sofia University in 1956 (a year earlier than his classmates). Immediately after thathewas admitted as a graduate student in the same department, but again for political reasons he was not allowed to continue his education. He had to work as a teacher for two years. With the very strong support of the mathematics professors (most importantly Prof. N. Obreshkov) Bl. Sendov was appointed in 1958 as an assistant professor in the Department of Mathematics. With his enormous energy, perseverance, strong will and ingenuity Bl. Sendov succeeded in developing a brilliant professional career. He was promoted to associate professor in 1963 and to full professor 1968. In 1964 he was awarded a Ph.D. -

Waste-Cluster Relationship on the Example of Regional Waste Landfill in Blagoevgrad
ECOLOGIA BALKANICA 2019, Special Edition 2 pp. 181-185 Waste-cluster Relationship on the Example of Regional Waste Landfill in Blagoevgrad Stefka K. Tsekova, Veselina H. Dalgacheva*, Nikolinka K. Atanasova South-West University “Neofit Rilski”, Faculty of Mathematics and Natural Sciences, Department of Geography, Ecology and Environmental Protection, 66 Ivan Mihailov Str., 2700 Blagoevgrad, BULGARIA *Corresponding author: [email protected] Abstract. Bulgaria is facing serious challenges about environmentally friendly way of waste management. Commissioning of the Regional Waste Management System - Blagoevgrad is going to ensure their environmentally friendly utilization and disposal, which is in close relation with the defined hierarchy in Bulgarian Waste management act - prevention, utilization, final disposal. The attempt to be created relation between regional waste management and cluster policy is related to the development of a cluster model, named by authors "Waste utilization and mitigation of climate change”. The implementation of the model will contribute to sustainable and efficient waste management in Blagoevgrad region, as well as in the territory of Bulgaria, in relation with the requirements of European legislation. In other side this contributing to the realization of the main Community priorities for Cohesion Policy: sustainable development and increasing the attractiveness of regions, by improving accessibility, ensuring adequate quality and level of services and preserving their environmental potential. Key words: regional waste management, cluster model, resource utilization, climate change, mitigation, Blagoevgrad, municipality. Introduction which includes municipalities from Environmentally friendly waste Blagoevgrad and Kyustendil province. management is a priority for the European (Ministry of environment and water, List of countries in transition, especially for the last Regional associations of waste management in 10-15 years. -

Republic of Bulgaria Ministry of Finance
REPUBLIC OF BULGARIA MINISTRY OF FINANCE ORDER № ZMF-70 Sofia, 01.02.2021 Pursuant to Art. 183, para. 1 in connection with para. 5 and Art. 184, item 1 of the Corporate Income Tax Act I ORDER: I determine a list of municipalities with unemployment with or over 25% higher than the national average for 2020. The order to be promulgated in the State gazette and to be published on the website of the Ministry of Finance. I assign the control over the execution of the order to the director of the Tax Policy Directorate. MINISTER: KIRIL ANANIEV LIST OF MUNICIPALITIES WITH AN UNEMPLOYMENT RATE OF 25 PERCENT EQUAL TO OR HIGHER THAN THE NATIONAL AVERAGE FOR 2020 № per line District / Municipality Unemployment rate I. Blagoevgrad District 1. Bansko 12,70 2. Belitsa 38,42 3. Garmen 14,59 4. Kresna 11,27 5. Petrich 10,35 6. Razlog 12,95 7. Satovcha 14,09 8. Simitli 14,42 9. Strumyani 20,65 10. Hadzhidimovo 13,49 11. Yakoruda 30,78 II. Burgas District 1. Ruen 13,75 2. Sredets 16,18 3. Sungurlare 10,90 III. Varna District 1. Avren 13,28 2. Byala 9,98 3. Vetrino 12,24 4. Valchi dol 20,75 5. Dolni Chiflik 14,94 6. Dalgopol 20,80 7. Provadiya 10,42 8. Suvorovo 11,39 IV. Veliko Tarnovo District 1. Elena 11,58 2. Zlataritsa 19,27 3. Polski Trambesh 12,39 4. Strazhitsa 17,09 5. Suhindol 14,52 V. Vidin District 1. Belogradchik 18,55 2. Boynitsa 14,22 3. -
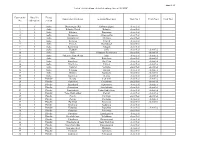
Annex 14 Consecutive No No of the Railway Line Energy Section from Station/Block Post to Station/Block Post Track No 1 Track No
Annex 14 List of electrified/non-electrified railway lines of SE NRIC Consecutive No of the Energy from station/block post to station/block post Track No 1 Track No 2 Track No 3 No railway line section 1 1 Sofia Dimitrovgrad RS Kalotina Zapad electrified 2 1 Sofia Kalotina Zapad Kalotina electrified 3 1 Sofia Kalotina Dragoman electrified 4 1 Sofia Dragoman Aldomirovtsi electrified 5 1 Sofia Aldomirovtsi Slivnitsa electrified 6 1 Sofia Slivnitsa Petarch electrified 7 1 Sofia Petarch Kostinbrod electrified 8 1 Sofia Kostinbrod Voluyak electrified 9 1 Sofia Voluyak Sofia electrified electrified 10 1 Sofia Sofia Poduyane Patnicheska electrified electrified 11 1 Sofia Poduyane Patnicheska Iskar electrified electrified 12 1 Sofia Iskar Kazichene electrified electrified 13 1 Sofia Kazichene Elin Pelin electrified electrified 14 1 Sofia Elin Pelin Vakarel electrified electrified 15 1 Sofia Vakarel Verinsko electrified electrified 16 1 Sofia Verinsko Ihtiman electrified electrified 17 1 Sofia Ihtiman Kostenets electrified electrified 18 1 Sofia Kostenets Belovo electrified electrified 19 1 Plovdiv Belovo Septemvri electrified electrified 20 1 Plovdiv Septemvri Pazardzhik electrified electrified 21 1 Plovdiv Pazardzhik Ognyanovo electrified electrified 22 1 Plovdiv Ognyanovo Stamboliyski electrified electrified 23 1 Plovdiv Stamboliyski Todor Kableshkov electrified electrified 24 1 Plovdiv Todor Kableshkov Plovdiv electrified electrified 25 1 Plovdiv Plovdiv Por Iztok electrified electrified 26 1 Plovdiv Plovdiv Por Iztok electrified electrified -

Kyustendil District
Kyustendil District Treklyano > Population (2015) 126,992 > Area (sq. km) 3,051.5 KYUSTENDIL Bobov dol SAPAREVA BANYA > Number of settlements 183 NEVESTINO DUPNITSA > Share of urban population (%) 69.1 BOBOSHEVO RILA KOCHERINOVO Overview lthough living conditions and social inclusion have deteriorate. The educational system has been successful in gradually been improving, salaries in the district have enrolling a great part of those subject to compulsory edu- Astayed low, while a considerable part of total households’ cation, though quality of education has been relatively low. incomes was generated by pensions. In 2015, unemploy- There has been a certain shortage of specialist doctors in ment fell and employment rose for the first time after the the district but the number of hospital beds is sufficient and crisis; both indicators, however, were still below the national the share of health-insured persons is high. Kyustendil has average levels. Kyustendil is among the districts which have received the second lowest rating after Sofia (capital city) for attracted least FDI so far and whose municipalities have uti- security and justice because of high crime rates, slow justice lized the least amount of EU funds. The rate of local taxes administration, and low crime clearance rate. In the “Envi- and fees was among the lowest in the country, yet, the ab- ronment” category, the district is characterized by good sence of transparency of local administrations has made the connectivity to public sewerage systems and wastewater district less attractive in comparison with others. treatment plants on one hand, and, by strongly polluted air, As a result of the unfavorable demographic tendencies, the on the other. -

Bulgaria 2000
TEAM FOR THE PREPARATION OF NATIONAL HUMAN DEVELOPMENT REPORT BULGARIA 2000 National Coordinator Adviser, Human Development Strategy Unit Dr. Andrey Ivanov Gerardo Berthin Contributors Dr. Antony Todorov, Dr. Belin Mollov, Dr. Dotcho Mihaylov, Dr. Georgi Ganev, Dr. Julia Spiridonova, Dr. Mikaela Vazharova, Dr. Vassil Marinov and Luchesar Bogdanov Statistical Team Micho Chipev, Prof. Yordan Venedikov, Sergey Tzvetarski, Stoyan Tzvetkov and Todor Todorov STRATEGIC PARTNERSHIPS OF NATIONAL HUMAN DEVELOPMENT REPORT BULGARIA 2000 National Statistical Institute National Center for Regional Development and Housing Policy National Association of Municipalities in the Republic of Bulgaria Friedrich Ebert Foundation, Sofia-Bulgaria ADVISORY COMMITTEE OF NATIONAL HUMAN DEVELOPMENT REPORT BULGARIA 2000 Svetlana Alexandrova, New Bulgarian University; Friedrich Bauersachs, Institute for Market Economics; Bisserka Benisheva, Ministry of Foreign Affairs; Mark Bossani, Ethnic Initiative for Human Rights Foundation; Vincenzo Celeste, Embassy of Italy; Ginka Chavdarova, National Association of Municipalities; Vera Dakova, Ideas in Process; Romain Darbelley, Embassy of Switzer- land; Hristo Hristozov, European Law Society; Pentcho Houbtchev, Friedrich Ebert Foundation; Ginka Kapitanova, Foundation for Local Government Reform; Christos Makridis, European Union Delegation; Fernando Nogales, Embassy of Spain; Jorge Nieto, European Union Delegation; Ivanka Petkova, Institute for Economic Policy; Kaye Pyle, United States Agency for International Development;Valery -

The Cult of Saints-Healers – an Alternative and Opposition to the Official Medicine in Medieval Bulgaria
JAHR Vol. 4 No. 7 2013 Review article Nadezhda Amudzhieva*, Pavel Tsvetkov** The cult of saints-healers – an alternative and opposition to the official medicine in medieval Bulgaria ABSTRACT Medieval Bulgarian medicine from the IX-XV c. was characterized by the low occurrence of medical services, by their inaccessibility, as well as by the widespread disappointment in learned physicians. This led to the search for alternative healing practices. Different means and methods were developed for filling up the deficit of healthcare services: • Self-treatment and self-proclaimed healers, • Healing through sacred objects, • Faith in saints and their relics. Evidence of the existence of a Bulgarian tradition of healing practices can be found in the considerable number of medical medieval works of utilitarian application, such as manuals, intended to be used by both – healers and patients. Pagan practices of worshipping magic items were transformed into the worship of objects of religious function – the cross, the Scriptures, holy water, holy oil, icons, etc., to which miracu- lous healing and saving powers were attributed. The cult of saints is highly utilitarian and focuses on the meeting of health care needs. Thus a parallel between self-proclaimed healers and saints was drawn, as saints were also believed to have been able to cure the faithful through God’s power. This cult has two aspects: • their supernatural powers, which the Saints had while still alive and • the miracles, associated with their relics. In the primitive medieval health care system, primary medical practice was not entrusted to the medical professionals, but to the saints-healers and their relics. -

Bulgarian Art and Culture
_ Bulgarian Art and Culture Historical and Contemporary Perspectives Fulbright Hays Seminar: Bulgaria [Summer 2004] Nancy Hart Assistant Professor of Art Ferrum College 40 Wiley Drive, Vaughn 200 Ferrum, Virginia 24088 540.365.4357 [email protected] _ Table of Contents Introduction 1 Map of Bulgaria 3 Introductory Text 4 Summary of Curriculum Projects 7 Project 1: Beyond the Expected: Travel and Tourism Poster 9 Project 2: Explorations of National Identity: Stamp Design 11 Project 3: Visual Expressions of Bulgarian Music 13 Project 4: Bulgarian Poetry: Into the Visual 16 Background on Bulgaria 18 Art 19 Music & Folklore 26 Poetry 27 Conclusion 41 Bibliography & Resources 42 Acknowledgements 44 All photos by the author unless noted. What makes us feel even more proud is the fact that our heritage has not yet become a museum exponent: that folklore not only lives on the stage but also presents a living artistic treasury in which modern Bulgarian composers, poets, writers, and artists look for ideas, characters, motifs and inspiration. This statement, from the Historical Museum in Smolyan, Bulgaria, demonstrates the integration of the past and the present in contemporary Bulgarian art and culture. _Bulgaria Country name: Republic of Bulgaria Government type: parliamentary democracy Population: 7,517,973 (July 2004 est.) Ethnic groups: Bulgarian 83.9%, Turk 9.4%, Roma 4.7%, other 2% (including Macedonian, Armenian, Tatar, Circassian) (2001) Religions: Bulgarian Orthodox 82.6%, Muslim 12.2%, Roman Catholic 1.7%, Jewish 0.1%, Protestant, Gregorian-Armenian, and other 3.4% (1998) Languages: Bulgarian, secondary languages closely correspond to ethnic breakdown Literacy: 98.6% Source: http://www.cia.gov/cia/publications/factbook/geos/bu.html Hart 3 _ Introduction My fi rst insights into contemporary Bulgaria began in Austin, Texas with our pre-trip orientation conducted at the University of Texas at Austin at the Center for Russian, East European and Eurasian Studies.