Part IV Elasticity and Thermodynamics of Reversible Processes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

10-1 CHAPTER 10 DEFORMATION 10.1 Stress-Strain Diagrams And
EN380 Naval Materials Science and Engineering Course Notes, U.S. Naval Academy CHAPTER 10 DEFORMATION 10.1 Stress-Strain Diagrams and Material Behavior 10.2 Material Characteristics 10.3 Elastic-Plastic Response of Metals 10.4 True stress and strain measures 10.5 Yielding of a Ductile Metal under a General Stress State - Mises Yield Condition. 10.6 Maximum shear stress condition 10.7 Creep Consider the bar in figure 1 subjected to a simple tension loading F. Figure 1: Bar in Tension Engineering Stress () is the quotient of load (F) and area (A). The units of stress are normally pounds per square inch (psi). = F A where: is the stress (psi) F is the force that is loading the object (lb) A is the cross sectional area of the object (in2) When stress is applied to a material, the material will deform. Elongation is defined as the difference between loaded and unloaded length ∆푙 = L - Lo where: ∆푙 is the elongation (ft) L is the loaded length of the cable (ft) Lo is the unloaded (original) length of the cable (ft) 10-1 EN380 Naval Materials Science and Engineering Course Notes, U.S. Naval Academy Strain is the concept used to compare the elongation of a material to its original, undeformed length. Strain () is the quotient of elongation (e) and original length (L0). Engineering Strain has no units but is often given the units of in/in or ft/ft. ∆푙 휀 = 퐿 where: is the strain in the cable (ft/ft) ∆푙 is the elongation (ft) Lo is the unloaded (original) length of the cable (ft) Example Find the strain in a 75 foot cable experiencing an elongation of one inch. -

The Conformations of Cycloalkanes
The Conformations of Cycloalkanes Ring-containing structures are a common occurrence in organic chemistry. We must, therefore spend some time studying the special characteristics of the parent cycloalkanes. Cyclical connectivity imposes constraints on the range of motion that the atoms in rings can undergo. Cyclic molecules are thus more rigid than linear or branched alkanes because cyclic structures have fewer internal degrees of freedom (that is, the motion of one atom greatly influences the motion of the others when they are connected in a ring). In this lesson we will examine structures of the common ring structures found in organic chemistry. The first four cycloalkanes are shown below. cyclopropane cyclobutane cyclopentane cyclohexane The amount of energy stored in a strained ring is estimated by comparing the experimental heat of formation to the calculated heat of formation. The calculated heat of formation is based on the notion that, in the absence of strain, each –CH2– group contributes equally to the heat of formation, in line with the behavior found for the acyclic alkanes (i.e., open chains). Thus, the calculated heat of formation varies linearly with the number of carbon atoms in the ring. Except for the 6-membered ring, the experimental values are found to have a more positive heat of formation than the calculated value owning to ring strain. The plots of calculated and experimental enthalpies of formation and their difference (i.e., ring strain) are seen in the Figure. The 3-membered ring has about 27 kcal/mol of strain. It can be seen that the 6-membered ring possesses almost no ring strain. -

Comparing Models for Measuring Ring Strain of Common Cycloalkanes
The Corinthian Volume 6 Article 4 2004 Comparing Models for Measuring Ring Strain of Common Cycloalkanes Brad A. Hobbs Georgia College Follow this and additional works at: https://kb.gcsu.edu/thecorinthian Part of the Chemistry Commons Recommended Citation Hobbs, Brad A. (2004) "Comparing Models for Measuring Ring Strain of Common Cycloalkanes," The Corinthian: Vol. 6 , Article 4. Available at: https://kb.gcsu.edu/thecorinthian/vol6/iss1/4 This Article is brought to you for free and open access by the Undergraduate Research at Knowledge Box. It has been accepted for inclusion in The Corinthian by an authorized editor of Knowledge Box. Campring Models for Measuring Ring Strain of Common Cycloalkanes Comparing Models for Measuring R..ing Strain of Common Cycloalkanes Brad A. Hobbs Dr. Kenneth C. McGill Chemistry Major Faculty Sponsor Introduction The number of carbon atoms bonded in the ring of a cycloalkane has a large effect on its energy. A molecule's energy has a vast impact on its stability. Determining the most stable form of a molecule is a usefol technique in the world of chemistry. One of the major factors that influ ence the energy (stability) of cycloalkanes is the molecule's ring strain. Ring strain is normally viewed as being directly proportional to the insta bility of a molecule. It is defined as a type of potential energy within the cyclic molecule, and is determined by the level of "strain" between the bonds of cycloalkanes. For example, propane has tl1e highest ring strain of all cycloalkanes. Each of propane's carbon atoms is sp3-hybridized. -
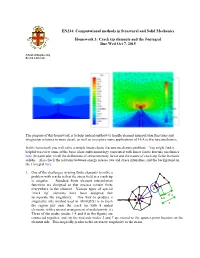
Crack Tip Elements and the J Integral
EN234: Computational methods in Structural and Solid Mechanics Homework 3: Crack tip elements and the J-integral Due Wed Oct 7, 2015 School of Engineering Brown University The purpose of this homework is to help understand how to handle element interpolation functions and integration schemes in more detail, as well as to explore some applications of FEA to fracture mechanics. In this homework you will solve a simple linear elastic fracture mechanics problem. You might find it helpful to review some of the basic ideas and terminology associated with linear elastic fracture mechanics here (in particular, recall the definitions of stress intensity factor and the nature of crack-tip fields in elastic solids). Also check the relations between energy release rate and stress intensities, and the background on the J integral here. 1. One of the challenges in using finite elements to solve a problem with cracks is that the stress field at a crack tip is singular. Standard finite element interpolation functions are designed so that stresses remain finite a everywhere in the element. Various types of special b c ‘crack tip’ elements have been designed that 3L/4 incorporate the singularity. One way to produce a L/4 singularity (the method used in ABAQUS) is to mesh L the region just near the crack tip with 8 noded elements, with a special arrangement of nodal points: (i) Three of the nodes (nodes 1,4 and 8 in the figure) are connected together, and (ii) the mid-side nodes 2 and 7 are moved to the quarter-point location on the element side. -

Today's Class Will Focus on the Conformations and Energies of Cycloalkanes
Name ______________________________________ Today's class will focus on the conformations and energies of cycloalkanes. Section 6.4 of the text provides an introduction. 1 Make a model of cyclopentane. Use the 2-piece "lockable" bond connectors — these rotate more easily than the smaller ones. You may need to twist them to "lock" so that they don't pop apart. The text shows two conformations of this ring — the envelope and the half-chair. Why is cyclopentane non-planar? The two conformations are below. In the envelope, 4 Cs are in a plane; in the half-chair 3 Cs are in a plane. Th first conformer is easy. To get your model to look like the second one, start with a planar ring, keep the "front" 3 Cs in the plane, and twist the "back" C–C bond so that one C goes above that plane and the other goes below. Add Hs to each of these structures — no wedges and dashes here — these are "perspective" drawings (like the drawings of chair cyclohexane rings on p 197). Just use your model and sketch the Hs where they appear to be. fast If we were to replace one H in each of these conformers with a methyl group, what do you think would be the most favorable position for it? Circle the one H on each structure above that you would replace with a CH3. In general, what happens to the ring C–C–C angles when a ring goes from planar to non-planar? Lecture outline Structures of cycloalkanes — C–C–C angles if planar: 60° 90° 108° 120° actual struct: planar |— slightly |— substantially non-planar —| non-planar —> A compound is strained if it is destabilized by: abnormal bond angles — van der Waals repulsions — eclipsing along σ-bonds — A molecule's strain energy (SE) is the difference between the enthalpy of formation of the compound of interest and that of a hypothetical strain-free compound that has the same atoms connected in exactly the same way. -

Cycloalkanes, Cycloalkenes, and Cycloalkynes
CYCLOALKANES, CYCLOALKENES, AND CYCLOALKYNES any important hydrocarbons, known as cycloalkanes, contain rings of carbon atoms linked together by single bonds. The simple cycloalkanes of formula (CH,), make up a particularly important homologous series in which the chemical properties change in a much more dramatic way with increasing n than do those of the acyclic hydrocarbons CH,(CH,),,-,H. The cyclo- alkanes with small rings (n = 3-6) are of special interest in exhibiting chemical properties intermediate between those of alkanes and alkenes. In this chapter we will show how this behavior can be explained in terms of angle strain and steric hindrance, concepts that have been introduced previously and will be used with increasing frequency as we proceed further. We also discuss the conformations of cycloalkanes, especially cyclo- hexane, in detail because of their importance to the chemistry of many kinds of naturally occurring organic compounds. Some attention also will be paid to polycyclic compounds, substances with more than one ring, and to cyclo- alkenes and cycloalkynes. 12-1 NOMENCLATURE AND PHYSICAL PROPERTIES OF CYCLOALKANES The IUPAC system for naming cycloalkanes and cycloalkenes was presented in some detail in Sections 3-2 and 3-3, and you may wish to review that ma- terial before proceeding further. Additional procedures are required for naming 446 12 Cycloalkanes, Cycloalkenes, and Cycloalkynes Table 12-1 Physical Properties of Alkanes and Cycloalkanes Density, Compounds Bp, "C Mp, "C diO,g ml-' propane cyclopropane butane cyclobutane pentane cyclopentane hexane cyclohexane heptane cycloheptane octane cyclooctane nonane cyclononane "At -40". bUnder pressure. polycyclic compounds, which have rings with common carbons, and these will be discussed later in this chapter. -

Cross-Linked Polymers and Rubber Elasticity Chapter 9 (Sperling)
Cross-linked Polymers and Rubber Elasticity Chapter 9 (Sperling) • Definition of Rubber Elasticity and Requirements • Cross-links, Networks, Classes of Elastomers (sections 1-3, 16) • Simple Theory of Rubber Elasticity (sections 4-8) – Entropic Origin of Elastic Retractive Forces – The Ideal Rubber Behavior • Departures from the Ideal Rubber Behavior (sections 9-11) – Non-zero Energy Contribution to the Elastic Retractive Forces – Stress-induced Crystallization and Limited Extensibility of Chains (How to make better elastomers: High Strength and High Modulus) – Network Defects (dangling chains, loops, trapped entanglements, etc..) – Semi-empirical Mooney-Rivlin Treatment (Affine vs Non-Affine Deformation) Definition of Rubber Elasticity and Requirements • Definition of Rubber Elasticity: Very large deformability with complete recoverability. • Molecular Requirements: – Material must consist of polymer chains. Need to change conformation and extension under stress. – Polymer chains must be highly flexible. Need to access conformational changes (not w/ glassy, crystalline, stiff mat.) – Polymer chains must be joined in a network structure. Need to avoid irreversible chain slippage (permanent strain). One out of 100 monomers must connect two different chains. Connections (covalent bond, crystallite, glassy domain in block copolymer) Cross-links, Networks and Classes of Elastomers • Chemical Cross-linking Process: Sol-Gel or Percolation Transition • Gel Characteristics: – Infinite Viscosity – Non-zero Modulus – One giant Molecule – Solid -

Chapter 10: Elasticity and Oscillations
Chapter 10 Lecture Outline 1 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Chapter 10: Elasticity and Oscillations •Elastic Deformations •Hooke’s Law •Stress and Strain •Shear Deformations •Volume Deformations •Simple Harmonic Motion •The Pendulum •Damped Oscillations, Forced Oscillations, and Resonance 2 §10.1 Elastic Deformation of Solids A deformation is the change in size or shape of an object. An elastic object is one that returns to its original size and shape after contact forces have been removed. If the forces acting on the object are too large, the object can be permanently distorted. 3 §10.2 Hooke’s Law F F Apply a force to both ends of a long wire. These forces will stretch the wire from length L to L+L. 4 Define: L The fractional strain L change in length F Force per unit cross- stress A sectional area 5 Hooke’s Law (Fx) can be written in terms of stress and strain (stress strain). F L Y A L YA The spring constant k is now k L Y is called Young’s modulus and is a measure of an object’s stiffness. Hooke’s Law holds for an object to a point called the proportional limit. 6 Example (text problem 10.1): A steel beam is placed vertically in the basement of a building to keep the floor above from sagging. The load on the beam is 5.8104 N and the length of the beam is 2.5 m, and the cross-sectional area of the beam is 7.5103 m2. -
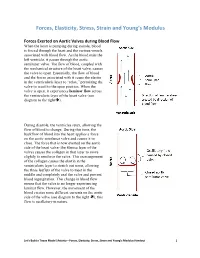
Forces, Elasticity, Stress, Strain and Young's Modulus Handout
Forces, Elasticity, Stress, Strain and Young’s Modulus Forces Exerted on Aortic Valves during Blood Flow When the heart is pumping during systole, blood is forced through the heart and the various vessels associated with blood flow. As the blood exits the left ventricle, it passes through the aortic semilunar valve. The flow of blood, coupled with the mechanical structure of the heart valve, causes the valve to open. Essentially, the flow of blood and the forces associated with it cause the elastin in the ventricularis layer to “relax,” permitting the valve to recoil to the open position. When the valve is open, it experiences laminar flow across the ventricularis layer of the heart valve (see diagram to the right). During diastole, the ventricles relax, allowing the flow of blood to change. During this time, the backflow of blood into the heart applies a force on the aortic semilunar valve and causes it to close. The force that is now exerted on the aortic side of the heart valve (the fibrosa layer of the valve) causes the collagen in that layer to move slightly to reinforce the valve. This rearrangement of the collagen causes the elastin in the ventricularis layer to stretch out some, allowing the three leaflets of the valve to meet in the middle and completely seal the valve and prevent blood regurgitation. This change in blood flow means that the valve is no longer experiencing laminar flow. However, the movement of the blood creates some different currents on the aortic side of the valve (see diagram to the right ); this flow is oscillatory in nature. -

Are the Most Well Studied of All Ring Systems. They Have a Limited Number Of, Almost Strain Free, Conformations
Chem 201/Beauchamp Topic 6, Conformations (cyclohexanes) 1 Cyclohexanes Cyclohexane rings (six atom rings in general) are the most well studied of all ring systems. They have a limited number of, almost strain free, conformations. Because of their well defined conformational shapes, they are frequently used to study effects of orientation or steric effects when studying chemical reactions. Additionally, six atom rings are the most commonly encountered rings in nature. Cyclohexane structures do not choose to be flat. Slight twists at each carbon atom allow cyclohexane rings to assume much more comfortable conformations, which we call chair conformations. (Chairs even sound comfortable.) The chair has an up and down shape all around the ring, sort of like the zig-zag shape seen in straight chains (...time for models!). C C C C C C lounge chair - used to kick back and relax while you study your organic chemistry chair conformation Cyclohexane rings are flexible and easily allow partial rotations (twists) about the C-C single bonds. There is minimal angle strain since each carbon can approximately accommodate the 109o of the tetrahedral shape. Torsional strain energy is minimized in chair conformations since all groups are staggered relative to one another. This is easily seen in a Newman projection perspective. An added new twist to our Newman projections is a side-by-side view of parallel single bonds. If you look carefully at the structure above or use your model, you should be able to see that a parallel relationship exists for all C-C bonds across the ring from one another. -

Thermodynamic Potential of Free Energy for Thermo-Elastic-Plastic Body
Continuum Mech. Thermodyn. (2018) 30:221–232 https://doi.org/10.1007/s00161-017-0597-3 ORIGINAL ARTICLE Z. Sloderbach´ · J. Paja˛k Thermodynamic potential of free energy for thermo-elastic-plastic body Received: 22 January 2017 / Accepted: 11 September 2017 / Published online: 22 September 2017 © The Author(s) 2017. This article is an open access publication Abstract The procedure of derivation of thermodynamic potential of free energy (Helmholtz free energy) for a thermo-elastic-plastic body is presented. This procedure concerns a special thermodynamic model of a thermo-elastic-plastic body with isotropic hardening characteristics. The classical thermodynamics of irre- versible processes for material characterized by macroscopic internal parameters is used in the derivation. Thermodynamic potential of free energy may be used for practical determination of the level of stored energy accumulated in material during plastic processing applied, e.g., for industry components and other machinery parts received by plastic deformation processing. In this paper the stored energy for the simple stretching of austenitic steel will be presented. Keywords Free energy · Thermo-elastic-plastic body · Enthalpy · Exergy · Stored energy of plastic deformations · Mechanical energy dissipation · Thermodynamic reference state 1 Introduction The derivation of thermodynamic potential of free energy for a model of the thermo-elastic-plastic body with the isotropic hardening is presented. The additive form of the thermodynamic potential is assumed, see, e.g., [1–4]. Such assumption causes that the influence of plastic strains on thermo-elastic properties of the bodies is not taken into account, meaning the lack of “elastic–plastic” coupling effects, see Sloderbach´ [4–10]. -

Physical Organic Chemistry
PHYSICAL ORGANIC CHEMISTRY Yu-Tai Tao (陶雨台) Tel: (02)27898580 E-mail: [email protected] Website:http://www.sinica.edu.tw/~ytt Textbook: “Perspective on Structure and Mechanism in Organic Chemistry” by F. A. Corroll, 1998, Brooks/Cole Publishing Company References: 1. “Modern Physical Organic Chemistry” by E. V. Anslyn and D. A. Dougherty, 2005, University Science Books. Grading: One midterm (45%) one final exam (45%) and 4 quizzes (10%) homeworks Chap.1 Review of Concepts in Organic Chemistry § Quantum number and atomic orbitals Atomic orbital wavefunctions are associated with four quantum numbers: principle q. n. (n=1,2,3), azimuthal q.n. (m= 0,1,2,3 or s,p,d,f,..magnetic q. n. (for p, -1, 0, 1; for d, -2, -1, 0, 1, 2. electron spin q. n. =1/2, -1/2. § Molecular dimensions Atomic radius ionic radius, ri:size of electron cloud around an ion. covalent radius, rc:half of the distance between two atoms of same element bond to each other. van der Waal radius, rvdw:the effective size of atomic cloud around a covalently bonded atoms. - Cl Cl2 CH3Cl Bond length measures the distance between nucleus (or the local centers of electron density). Bond angle measures the angle between lines connecting different nucleus. Molecular volume and surface area can be the sum of atomic volume (or group volume) and surface area. Principle of additivity (group increment) Physical basis of additivity law: the forces between atoms in the same molecule or different molecules are very “short range”. Theoretical determination of molecular size:depending on the boundary condition.