GAZE and AUTONOMIC INNERVATION DISORDERS Eye64 (1)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Treacher Collins Prize Essay the Significance of Nystagmus
Eye (1989) 3, 816--832 Treacher Collins Prize Essay The Significance of Nystagmus NICHOLAS EVANS Norwich Introduction combined. The range of forms it takes, and Ophthalmology found the term v!to"[<xy!too, the circumstances in which it occurs, must be like many others, in classical Greece, where it compared and contrasted in order to under described the head-nodding of the wined and stand the relationships between nystagmus of somnolent. It first acquired a neuro-ophthal different aetiologies. An approach which is mological sense in 1822, when it was used by synthetic as well as analytic identifies those Goodl to describe 'habitual squinting'. Since features which are common to different types then its meaning has been refined, and much and those that are distinctive, and helps has been learned about the circumstances in describe the relationship between eye move which the eye oscillates, the components of ment and vision in nystagmus. nystagmus, and its neurophysiological, Nystagmus is not properly a disorder of eye neuroanatomic and neuropathological corre movement, but one of steady fixation, in lates. It occurs physiologically and pathologi which the relationship between eye and field cally, alone or in conjunction with visual or is unstable. The essential significance of all central nervous system pathology. It takes a types of nystagmus is the disturbance in this variety of different forms, the eyes moving relationship between the sensory and motor about one or more axis, and may be conjugate ends of the visual-oculomotor axis. Optimal or dysjugate. It can be modified to a variable visual performance requires stability of the degree by external (visual, gravitational and image on the retina, and vision is inevitably rotational) and internal (level of awareness affected by nystagmus. -

Stroke and Transient Ischaemic Attacks
53454ournal ofNeurology, Neurosurgery, and Psychiatry 1994;57:534-543 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.57.5.534 on 1 May 1994. Downloaded from NEUROLOGICAL MANAGEMENT Stroke and transient ischaemic attacks Peter Humphrey The management of stroke is expensive and ANATOMY accounts for about 5% of NHS hospital costs. Carotid v vertebrobasilar arterial territory Stroke is the commonest cause of severe Carotid-Classifying whether a stroke is in the physical disability. About 100 000 people territory of the carotid or vertebrobasilar suffer a first stroke each year in England and arteries is important, especially if the patient Wales. It is important to emphasise that makes a good recovery. Carotid endarterec- around 20-25% of all strokes affects people tomy is of proven value in those with carotid under 65 years of age. The annual incidence symptoms. Carotid stroke usually produces of stroke is two per 1000.' About 10% of all hemiparesis, hemisensory loss, or dysphasia. patients suffer a recurrent stroke within one Apraxia and visuospatial problems may also year. The prevalence of stroke shows that occur. If there is a severe deficit, there may there are 250 000 in England and Wales. also be an homonymous hemianopia and gaze Each year approximately 60 000 people are palsy. Episodes of amaurosis fugax or central reported to die of stroke; this represents retinal artery occlusion are also carotid about 12% of all deaths. Only ischaemic events. heart disease and cancer account for more Homer's syndrome can occur because of deaths. This means that in an "average" dis- damage to the sympathetic fibres in the trict health authority of 250 000 people, there carotid sheath. -

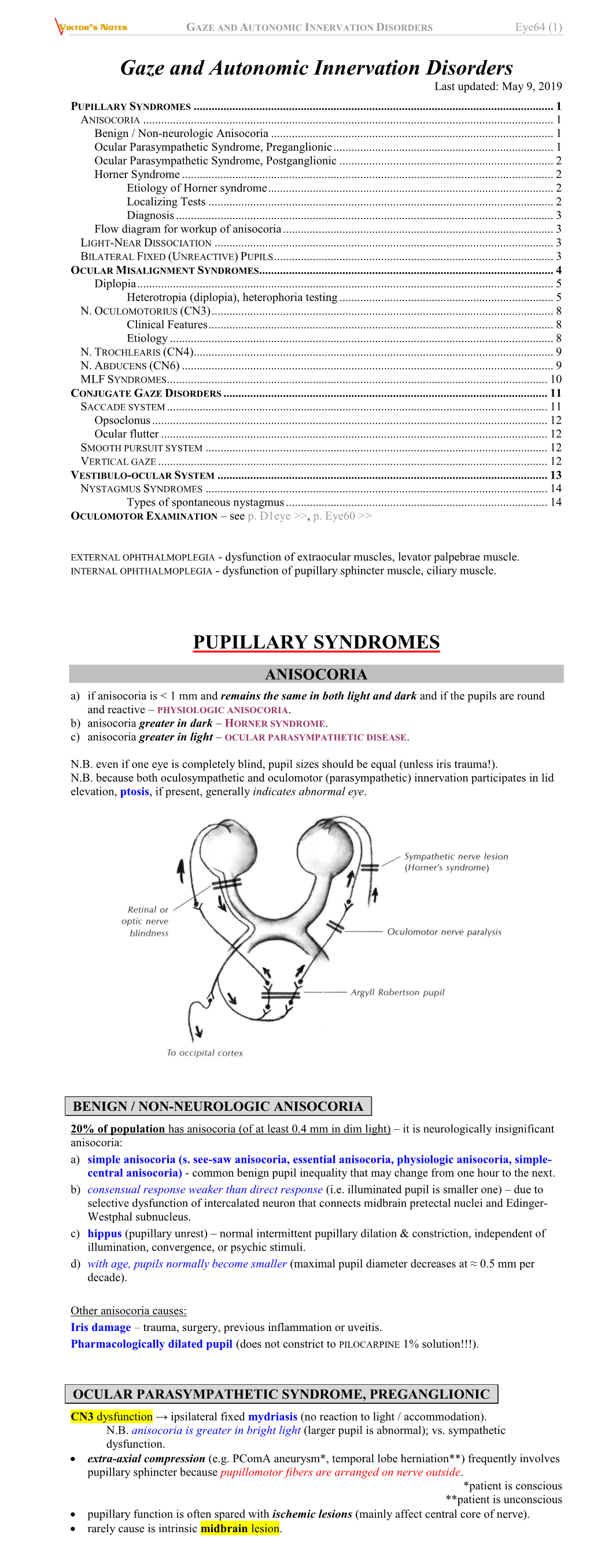
Pupillary Disorders LAURA J
13 Pupillary Disorders LAURA J. BALCER Pupillary disorders usually fall into one of three major cat- cortex generally do not affect pupillary size or reactivity. egories: (1) abnormally shaped pupils, (2) abnormal pupillary Efferent parasympathetic fibers, arising from the Edinger– reaction to light, or (3) unequally sized pupils (anisocoria). Westphal nucleus, exit the midbrain within the third nerve Occasionally pupillary abnormalities are isolated findings, (efferent arc). Within the subarachnoid portion of the third but in many cases they are manifestations of more serious nerve, pupillary fibers tend to run on the external surface, intracranial pathology. making them more vulnerable to compression or infiltration The pupillary examination is discussed in detail in and less susceptible to vascular insult. Within the anterior Chapter 2. Pupillary neuroanatomy and physiology are cavernous sinus, the third nerve divides into two portions. reviewed here, and then the various pupillary disorders, The pupillary fibers follow the inferior division into the orbit, grouped roughly into one of the three listed categories, are where they then synapse at the ciliary ganglion, which lies discussed. in the posterior part of the orbit between the optic nerve and lateral rectus muscle (Fig. 13.3). The ciliary ganglion issues postganglionic cholinergic short ciliary nerves, which Neuroanatomy and Physiology initially travel to the globe with the nerve to the inferior oblique muscle, then between the sclera and choroid, to The major functions of the pupil are to vary the quantity of innervate the ciliary body and iris sphincter muscle. Fibers light reaching the retina, to minimize the spherical aberra- to the ciliary body outnumber those to the iris sphincter tions of the peripheral cornea and lens, and to increase the muscle by 30 : 1. -

Article • a Case Series in Optometric Management of Diverse Vertical
Article • A Case Series in Optometric Management of Diverse Vertical Deviations Darah McDaniel-Chandler, OD • Southern College of Optometry Memphis, Tennessee ABSTRACT Background: Vertical deviations present in diverse patient populations with a multitude of puzzling symptoms and complaints. Many patients with vertical deviations have visited numerous doctors looking for an explanation for their symptoms of dizziness, headaches, motion sickness, and double vision. Vertical deviations may be apparent in the clinical optometric exam sequence, but at other times, additional testing must be performed to uncover a vertical heterophoria, including fixation disparity, vertical vergences, a period of diagnostic occlusion, or Maddox rod. Case Report: Three case reports are reviewed with diverse presentations of vertical deviations. The first case report outlines Patient A, a 51-year-old female who presented with dizziness along with a latent hyperphoria that was not apparent on the initial clinical examination. The second case report outlines Patient B, a 52-year-old female who presented with a longstanding large-angle vertical strabismus with strabismic amblyopia in the right eye. The third case report outlines Patient C, a 61-year-old female who presented with intermittent vertical diplopia following a cerebrovascular accident. The three cases all underwent vision therapy or vision therapy in combination with prismatic correction, and all three cases experienced symptom reduction following treatment with optometric vision rehabilitation. Conclusion: -

Cranial Nerve Palsy
Cranial Nerve Palsy What is a cranial nerve? Cranial nerves are nerves that lead directly from the brain to parts of our head, face, and trunk. There are 12 pairs of cranial nerves and some are involved in special senses (sight, smell, hearing, taste, feeling) while others control muscles and glands. Which cranial nerves pertain to the eyes? The second cranial nerve is called the optic nerve. It sends visual information from the eye to the brain. The third cranial nerve is called the oculomotor nerve. It is involved with eye movement, eyelid movement, and the function of the pupil and lens inside the eye. The fourth cranial nerve is called the trochlear nerve and the sixth cranial nerve is called the abducens nerve. They each innervate an eye muscle involved in eye movement. The fifth cranial nerve is called the trigeminal nerve. It provides facial touch sensation (including sensation on the eye). What is a cranial nerve palsy? A palsy is a lack of function of a nerve. A cranial nerve palsy may cause a complete or partial weakness or paralysis of the areas served by the affected nerve. In the case of a cranial nerve that has multiple functions (such as the oculomotor nerve), it is possible for a palsy to affect all of the various functions or only some of the functions of that nerve. What are some causes of a cranial nerve palsy? A cranial nerve palsy can occur due to a variety of causes. It can be congenital (present at birth), traumatic, or due to blood vessel disease (hypertension, diabetes, strokes, aneurysms, etc). -

"Nystagmus Testing in Intoxicated Individuals," Citek
ISSUE HIGHLIGHT Nystagmus testing in intoxicated individuals Karl Citek, O.D., Ph.D.,a Bret Ball, O.D.,a and Dale A. Rutledge, Lieutenantb aCollege of Optometry, Pacific University, Forest Grove, Oregon and bthe Oregon State Police, Wilsonville, Oregon Background: Law enforcement officers routinely conduct psy- n the United States, drivers impaired* by alcohol and/or chophysical tests to determine if an impaired driver may be drugs are responsible for more than 16,000 deaths, one intoxicated or in need of medical assistance. Testing includes million injuries, and $45 billion in costs annually.1 As assessment of eye movements, using the Horizontal Gaze Nys- I tagmus (HGN) and Vertical Gaze Nystagmus (VGN) tests, which part of the attempt to reduce these human and economic are conducted at roadside by patrol officers. These tests pre- tolls, law enforcement officers routinely conduct tests of eye viously have been validated when the subject is placed in a movements to determine if a driver is under the influence standing posture with head upright. However, certain condi- of alcohol or other drugs. Alcohol, other central nervous sys- tions require that the subject be tested while seated or supine. Under these conditions, Positional Alcohol Nystagmus (PAN) tem (CNS)-depressant drugs, inhalants, and phencyclidine could be induced and mistaken for HGN or VGN. (PCP) and its analogs will affect the neural centers in the Methods: The study was conducted at law enforcement train- brainstem and cerebellum, which control eye movements, ing academy alcohol workshops in the Pacific Northwest. as well as other motor, sensory, and cognitive integration Ninety-six volunteer drinkers were tested when sober and areas of the brain. -

Taking the Mystery out of Abnormal Pupils
Taking the mystery out of abnormal pupils No financial disclosures Course Title: Taking the mystery out [email protected] of abnormal pupils Lecturer: Brad Sutton, OD, FAAO Clinical Professor IU School of Optometry . •Review of Anatomy Iris anatomy Iris sphincter Iris dilator Parasympathetic pathway Sympathetic pathway Parasympathetic Pathway Parasympathetic Pathway Light stimulates the retina then impulse Four neuron arc travels with the ganglion cells through the Retina to the pretectal nucleus in the chiasm into the optic tracts. 80% go to the midbrain (1) LGN , 20% to the pretectal nuclei.They Pretectal nucleus to the EW nucleus (2) then hemidecussate and terminate at the EW nucleus EW nucleus to the ciliary ganglion (3) Ciliary ganglion to the iris sphincter with short ciliary nerves (4) 1 Points of Interest Sympathetic Pathway Within the second order neuron there are Three neuron arc 30 near response fibers for every light Posterior hypothalamus to ciliospinal response fiber. This allows for light - near center of Budge ( C8 - T2 ). (1) dissociation. Center of Budge to the superior cervical The third order neuron runs with cranial ganglion in the neck (2) nerve III from the brain stem to the ciliary Superior cervical ganglion to the dilator ganglion. Superficially located prior to the muscle (3) cavernous sinus. Points of Interest Second order neuron runs along the surface of the lung, can be affected by a Pancoast tumor Third order neuron runs with the carotid artery then with the ophthalmic division of cranial nerve V 2 APD Testing testing……………….AKA……… … APD / reverse APD Direct and consensual response Which is the abnormal pupil ? Very simple rule. -

Clinicalguidelines
Postgrad MedJ7 1995; 71: 577-584 © The Fellowship of Postgraduate Medicine, 1995 Clinical guidelines Postgrad Med J: first published as 10.1136/pgmj.71.840.577 on 1 October 1995. Downloaded from Management of transient ischaemic attacks and stroke PRD Humphrey Summary The management of stroke and transient ischaemic attacks (TIAs) consumes The management of stroke and about 500 of NHS hospital costs. Stroke is the commonest cause of severe transient ischaemic attacks physical disability with an annual incidence of two per 1000.1 In an 'average' (TIAs) has changed greatly in the district general hospital of 250 000 people there will be 500 first strokes in one last two decades. The importance year with a prevalence of about 1500. TIAs are defined as acute, focal of good blood pressure control is neurological symptoms, resulting from vascular disease which resolve in less the hallmark of stroke preven- than 24 hours. The incidence of a TIA is 0.5 per 1000. tion. Large multicentre trials Stroke is not a diagnosis. It is merely a description of a symptom complex have proven beyond doubt the thought to have a vascular aetiology. It is important to classify stroke according value of aspirin in TIAs, warfarin to the anatomy ofthe lesion, its timing, aetiology and pathogenesis. This will help in patients with atrial fibrillation to decide the most appropriate management. and embolic cerebrovascular symptoms, and carotid endarter- Classification of stroke ectomy in patients with carotid TIAs. There seems little doubt Many neurologists have described erudite vascular syndromes in the past. Most that patients managed in acute ofthese are oflittle practical use. -

Complex Strabismus and Syndromes
Complex Strabismus & Syndromes Some patients exhibit complex combinations of vertical, horizontal, and torsional strabismus. Dr. Shin treats patients with complex strabismus arising from, but not limited to, thyroid-related eye disease, stroke, or brain tumors as well as strabismic disorders following severe orbital and head trauma. The following paragraphs describe specific ocular conditions marked by complex strabismus. Duane Syndrome Duane syndrome represents a constellation of eye findings present at birth that results from an absent 6th cranial nerve nucleus and an aberrant branch of the 3rd cranial nerve that innervates the lateral rectus muscle. Duane syndrome most commonly affects the left eye of otherwise healthy females. Duane syndrome includes several variants of eye movement abnormalities. In the most common variant, Type I, the eye is unable to turn outward to varying degrees from the normal straight ahead position. In addition, when the patient tries to look straight ahead, the eyes may cross. This may lead a person with Duane syndrome to turn his/her head toward one side while viewing objects in front of them in order to better align the eyes. When the involved eye moves toward the nose, the eye retracts slightly back into the eye socket causing a narrowing of the opening between the eyelids. In Type II, the affected eye possesses limited ability to turn inward and is generally outwardly turning. In Type III, the eye has limited inward and outward movement. All three types are characterized by anomalous co-contraction of the medial and lateral rectus muscles, so when the involved eye moves towards the nose, the globe pulls back into the orbit and the vertical space between the eyelids narrows. -

Complete and Incomplete Forms of the Benign Disorder Characterised By
Br J Ophthalmol: first published as 10.1136/bjo.16.8.449 on 1 August 1932. Downloaded from THE BRITISH JOURNAL OF OPHTHALMOLOGY AUGUST, 1932 COMMUNICATIONS COMPLETE AND INCOMPLETE FORMS OF THE BENIGN DISORDER CHARACTERISED BY TONIC PUPILS AND ABSENT copyright. TENDON REFLEXES BY W. J. ADIE, M.D., F.R.C.P. PHYSICIAN, CHARING CROSS HOSPITAL AND ROYAL LONDON OPHTHALMIC HOSPITAL. OUT-PATIENT PHYSICIAN, THE NATIONAL HOSPITAL FOR NERVOUS DISEASES, QUEEN SQUARE http://bjo.bmj.com/ IN earlier papers on this subject it has been suggested that certain apparently dissimilar abnormal pupillary reactions formerly described as occurring in unrelated conditions are manifestations of the same disorder. The abnormal reactions are, on the one hand, the tonic pupillary reaction (syn. pupillotonia, myotonic reaction, tonic convergence reaction of pupils apparently inactive to light, on September 30, 2021 by guest. Protected Marcus's peculiar pupil phenomenon, non-luetic Argyll Robertson pupil, pseudo-Argyll Robertson pupil) and, on the other hand what may be called atypical phases of the tonic pupil. In the atypical phases tonic reactions are absent or difficult to detect and the state of the pupil in cases that present them is usually desig- nated by the term fixed pupil, ophthalmoplegia interna, iridoplegia or partial iridoplegia, of unknown origin. The disorder referred to, if the views expressed here are correct, may manifest itself in the following clinical forms: A. The complete form characterized by the presence in one or both eyes of the tonic convergence reaction in a pupil apparently Br J Ophthalmol: first published as 10.1136/bjo.16.8.449 on 1 August 1932. -

Supranuclear and Internuclear Ocular Motility Disorders
CHAPTER 19 Supranuclear and Internuclear Ocular Motility Disorders David S. Zee and David Newman-Toker OCULAR MOTOR SYNDROMES CAUSED BY LESIONS IN OCULAR MOTOR SYNDROMES CAUSED BY LESIONS OF THE MEDULLA THE SUPERIOR COLLICULUS Wallenberg’s Syndrome (Lateral Medullary Infarction) OCULAR MOTOR SYNDROMES CAUSED BY LESIONS OF Syndrome of the Anterior Inferior Cerebellar Artery THE THALAMUS Skew Deviation and the Ocular Tilt Reaction OCULAR MOTOR ABNORMALITIES AND DISEASES OF THE OCULAR MOTOR SYNDROMES CAUSED BY LESIONS IN BASAL GANGLIA THE CEREBELLUM Parkinson’s Disease Location of Lesions and Their Manifestations Huntington’s Disease Etiologies Other Diseases of Basal Ganglia OCULAR MOTOR SYNDROMES CAUSED BY LESIONS IN OCULAR MOTOR SYNDROMES CAUSED BY LESIONS IN THE PONS THE CEREBRAL HEMISPHERES Lesions of the Internuclear System: Internuclear Acute Lesions Ophthalmoplegia Persistent Deficits Caused by Large Unilateral Lesions Lesions of the Abducens Nucleus Focal Lesions Lesions of the Paramedian Pontine Reticular Formation Ocular Motor Apraxia Combined Unilateral Conjugate Gaze Palsy and Internuclear Abnormal Eye Movements and Dementia Ophthalmoplegia (One-and-a-Half Syndrome) Ocular Motor Manifestations of Seizures Slow Saccades from Pontine Lesions Eye Movements in Stupor and Coma Saccadic Oscillations from Pontine Lesions OCULAR MOTOR DYSFUNCTION AND MULTIPLE OCULAR MOTOR SYNDROMES CAUSED BY LESIONS IN SCLEROSIS THE MESENCEPHALON OCULAR MOTOR MANIFESTATIONS OF SOME METABOLIC Sites and Manifestations of Lesions DISORDERS Neurologic Disorders that Primarily Affect the Mesencephalon EFFECTS OF DRUGS ON EYE MOVEMENTS In this chapter, we survey clinicopathologic correlations proach, although we also discuss certain metabolic, infec- for supranuclear ocular motor disorders. The presentation tious, degenerative, and inflammatory diseases in which su- follows the schema of the 1999 text by Leigh and Zee (1), pranuclear and internuclear disorders of eye movements are and the material in this chapter is intended to complement prominent. -

Retina and Neuro Ophthalmology
Name : …………………………………………………………… Roll No. : ………………………………………………………… Invigilator's Signature : ……………………………………….. CS/B.OPTM/SEM-5/BO-504/2010-11 2010-11 OCULAR DISEASE - II POSTERIOR SEGMENT ( RETINA & NEURO-OPHTHALMOLOGY ) Time Allotted : 3 Hours Full Marks : 70 The figures in the margin indicate full marks. Candidates are required to give their answers in their own words as far as practicable. GROUP – A ( Multiple Choice Type Questions ) 1. Choose the correct alternatives for any ten of the following : 10 × 1 = 10 i) Which is not an important diagnostic criterion of giant cell arteritis ? http://www.makaut.com/ a) ESR > 70 mm/hour b) C-reactive protein > 2·45 mg/dl c) Jaw caludication d) Neck pain. ii) Unilateral blindness in a male child with massive exudation under the retina is most likely a case of a) Coat's disease b) Retinoblastoma c) Sturge-Weber syndrome d) Louis-Bar's syndrome. 5323 [ Turn over http://www.makaut.com/ CS/B.OPTM/SEM-5/BO-504/2010-11 iii) A retinal detachment patient mostly complains of a) pain b) good vision c) flashes and floaters d) diplopia. iv) In myasthenia gravis, during a diagnostic test, we use a drug called a) Piperazine citrate b) Azathioprim c) Edrophonium d) Carbamazepine. v) Sillicone oil is a/an a) aqueous substitute b) lens substitute c) vitreous substitute d) artificial lens. vi) In retinoblastoma, if the microscopical examination shows Flexner-Wintersteiner rosettes, it is considered to be a) highly malignant b) less malignant c) not malignant d) none of these. vii) Dyschromatopsia is the term for defective a) day vision b) night vision http://www.makaut.com/ c) colour vision d) light brightness sensitivity.