Cirrhosis a Patient Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chronic Viral Hepatitis in a Cohort of Inflammatory Bowel Disease
pathogens Article Chronic Viral Hepatitis in a Cohort of Inflammatory Bowel Disease Patients from Southern Italy: A Case-Control Study Giuseppe Losurdo 1,2 , Andrea Iannone 1, Antonella Contaldo 1, Michele Barone 1 , Enzo Ierardi 1 , Alfredo Di Leo 1,* and Mariabeatrice Principi 1 1 Section of Gastroenterology, Department of Emergency and Organ Transplantation, University “Aldo Moro” of Bari, 70124 Bari, Italy; [email protected] (G.L.); [email protected] (A.I.); [email protected] (A.C.); [email protected] (M.B.); [email protected] (E.I.); [email protected] (M.P.) 2 Ph.D. Course in Organs and Tissues Transplantation and Cellular Therapies, Department of Emergency and Organ Transplantation, University “Aldo Moro” of Bari, 70124 Bari, Italy * Correspondence: [email protected]; Tel.: +39-080-559-2925 Received: 14 September 2020; Accepted: 21 October 2020; Published: 23 October 2020 Abstract: We performed an epidemiologic study to assess the prevalence of chronic viral hepatitis in inflammatory bowel disease (IBD) and to detect their possible relationships. Methods: It was a single centre cohort cross-sectional study, during October 2016 and October 2017. Consecutive IBD adult patients and a control group of non-IBD subjects were recruited. All patients underwent laboratory investigations to detect chronic hepatitis B (HBV) and C (HCV) infection. Parameters of liver function, elastography and IBD features were collected. Univariate analysis was performed by Student’s t or chi-square test. Multivariate analysis was performed by binomial logistic regression and odds ratios (ORs) were calculated. We enrolled 807 IBD patients and 189 controls. Thirty-five (4.3%) had chronic viral hepatitis: 28 HCV (3.4%, versus 5.3% in controls, p = 0.24) and 7 HBV (0.9% versus 0.5% in controls, p = 0.64). -

Zoonotic Diseases Fact Sheet
ZOONOTIC DISEASES FACT SHEET s e ion ecie s n t n p is ms n e e s tio s g s m to a a o u t Rang s p t tme to e th n s n m c a s a ra y a re ho Di P Ge Ho T S Incub F T P Brucella (B. Infected animals Skin or mucous membrane High and protracted (extended) fever. 1-15 weeks Most commonly Antibiotic melitensis, B. (swine, cattle, goats, contact with infected Infection affects bone, heart, reported U.S. combination: abortus, B. suis, B. sheep, dogs) animals, their blood, tissue, gallbladder, kidney, spleen, and laboratory-associated streptomycina, Brucellosis* Bacteria canis ) and other body fluids causes highly disseminated lesions bacterial infection in tetracycline, and and abscess man sulfonamides Salmonella (S. Domestic (dogs, cats, Direct contact as well as Mild gastroenteritiis (diarrhea) to high 6 hours to 3 Fatality rate of 5-10% Antibiotic cholera-suis, S. monkeys, rodents, indirect consumption fever, severe headache, and spleen days combination: enteriditis, S. labor-atory rodents, (eggs, food vehicles using enlargement. May lead to focal chloramphenicol, typhymurium, S. rep-tiles [especially eggs, etc.). Human to infection in any organ or tissue of the neomycin, ampicillin Salmonellosis Bacteria typhi) turtles], chickens and human transmission also body) fish) and herd animals possible (cattle, chickens, pigs) All Shigella species Captive non-human Oral-fecal route Ranges from asymptomatic carrier to Varies by Highly infective. Low Intravenous fluids primates severe bacillary dysentery with high species. 16 number of organisms and electrolytes, fevers, weakness, severe abdominal hours to 7 capable of causing Antibiotics: ampicillin, cramps, prostration, edema of the days. -

Primary Biliary Cholangitis: a Brief Overview Justin S
REVIEW Primary Biliary Cholangitis: A Brief Overview Justin S. Louie,* Sirisha Grandhe,* Karen Matsukuma,† and Christopher L. Bowlus* Primary biliary cholangitis (PBC), previously referred to supported by the higher concordance of PBC in monozy- as primary biliary cirrhosis, is the most common chronic gotic compared with dizygotic twins.4 In addition, certain cholestatic autoimmune disease affecting adults in the human leukocyte antigen haplotypes have been associ- United States.1 It is characterized by a hallmark serologic ated with PBC, as well as variants at loci along the inter- signature, antimitochondrial antibody (AMA), and specific leukin-12 (IL-12) immunoregulatory pathway (IL-12A and bile duct pathology with progressive intrahepatic duct de- IL-12RB2 loci).5 struction leading to cholestasis. PBC is potentially fatal and can have both intrahepatic and extrahepatic complications. PATHOGENESIS EPIDEMIOLOGY The primary disease mechanism in PBC is thought to be T cell lymphocyte–mediated injury against intralobu- PBC affects all races and ethnicities; however, it is best lar biliary epithelial cells. This causes progressive destruc- studied in the Caucasian population. The condition pre- tion and eventual disappearance of the intralobular bile dominantly affects women older than 40 years, with a ducts. Molecular mimicry has been proposed as the ini- female/male ratio of 9:1.2 Although the incidence of PBC tiating event in the loss of tolerance primarily to mito- appears to be stable, the overall prevalence of the disease chondrial pyruvate dehydrogenase complex, E2, during is increasing.3 An individual’s genetic susceptibility, epige- which exogenous antigens evoke an immune response netic factors, and certain environmental triggers seem to that recognizes an endogenous (self) antigen inciting an play important roles. -
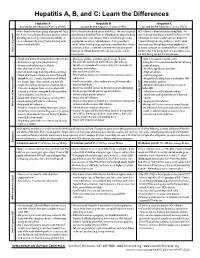
Hepatitis A, B, and C: Learn the Differences
Hepatitis A, B, and C: Learn the Differences Hepatitis A Hepatitis B Hepatitis C caused by the hepatitis A virus (HAV) caused by the hepatitis B virus (HBV) caused by the hepatitis C virus (HCV) HAV is found in the feces (poop) of people with hepa- HBV is found in blood and certain body fluids. The virus is spread HCV is found in blood and certain body fluids. The titis A and is usually spread by close personal contact when blood or body fluid from an infected person enters the body virus is spread when blood or body fluid from an HCV- (including sex or living in the same household). It of a person who is not immune. HBV is spread through having infected person enters another person’s body. HCV can also be spread by eating food or drinking water unprotected sex with an infected person, sharing needles or is spread through sharing needles or “works” when contaminated with HAV. “works” when shooting drugs, exposure to needlesticks or sharps shooting drugs, through exposure to needlesticks on the job, or from an infected mother to her baby during birth. or sharps on the job, or sometimes from an infected How is it spread? Exposure to infected blood in ANY situation can be a risk for mother to her baby during birth. It is possible to trans- transmission. mit HCV during sex, but it is not common. • People who wish to be protected from HAV infection • All infants, children, and teens ages 0 through 18 years There is no vaccine to prevent HCV. -

Active Peptic Ulcer Disease in Patients with Hepatitis C Virus-Related Cirrhosis: the Role of Helicobacter Pylori Infection and Portal Hypertensive Gastropathy
dore.qxd 7/19/2004 11:24 AM Page 521 View metadata, citation and similar papers at core.ac.uk ORIGINAL ARTICLE brought to you by CORE provided by Crossref Active peptic ulcer disease in patients with hepatitis C virus-related cirrhosis: The role of Helicobacter pylori infection and portal hypertensive gastropathy Maria Pina Dore MD PhD, Daniela Mura MD, Stefania Deledda MD, Emmanouil Maragkoudakis MD, Antonella Pironti MD, Giuseppe Realdi MD MP Dore, D Mura, S Deledda, E Maragkoudakis, Ulcère gastroduodénal évolutif chez les A Pironti, G Realdi. Active peptic ulcer disease in patients patients atteints de cirrhose liée au HCV : Le with hepatitis C virus-related cirrhosis: The role of Helicobacter pylori infection and portal hypertensive rôle de l’infection à Helicobacter pylori et de la gastropathy. Can J Gastroenterol 2004;18(8):521-524. gastropathie liée à l’hypertension portale BACKGROUND & AIM: The relationship between Helicobacter HISTORIQUE ET BUT : Le lien entre l’infection à Helicobacter pylori pylori infection and peptic ulcer disease in cirrhosis remains contro- et l’ulcère gastroduodénal dans la cirrhose reste controversé. Le but de la versial. The purpose of the present study was to investigate the role of présente étude est de vérifier le rôle de l’infection à H. pylori et de la gas- H pylori infection and portal hypertension gastropathy in the preva- tropathie liée à l’hypertension portale dans la prévalence de l’ulcère gas- lence of active peptic ulcer among dyspeptic patients with compen- troduodénal évolutif chez les patients dyspeptiques souffrant d’une sated hepatitis C virus (HCV)-related cirrhosis. -

Non-Alcoholic Fatty Liver Disease
Non-alcoholic fatty liver disease Description Non-alcoholic fatty liver disease (NAFLD) is a buildup of excessive fat in the liver that can lead to liver damage resembling the damage caused by alcohol abuse, but that occurs in people who do not drink heavily. The liver is a part of the digestive system that helps break down food, store energy, and remove waste products, including toxins. The liver normally contains some fat; an individual is considered to have a fatty liver (hepatic steatosis) if the liver contains more than 5 to 10 percent fat. The fat deposits in the liver associated with NAFLD usually cause no symptoms, although they may cause increased levels of liver enzymes that are detected in routine blood tests. Some affected individuals have abdominal pain or fatigue. During a physical examination, the liver may be found to be slightly enlarged. Between 7 and 30 percent of people with NAFLD develop inflammation of the liver (non- alcoholic steatohepatitis, also known as NASH), leading to liver damage. Minor damage to the liver can be repaired by the body. However, severe or long-term damage can lead to the replacement of normal liver tissue with scar tissue (fibrosis), resulting in irreversible liver disease (cirrhosis) that causes the liver to stop working properly. Signs and symptoms of cirrhosis, which get worse as fibrosis affects more of the liver, include fatigue, weakness, loss of appetite, weight loss, nausea, swelling (edema), and yellowing of the skin and whites of the eyes (jaundice). Scarring in the vein that carries blood into the liver from the other digestive organs (the portal vein) can lead to increased pressure in that blood vessel (portal hypertension), resulting in swollen blood vessels (varices) within the digestive system. -

Vaccinations for Adults with Chronic Liver Disease Or Infection
Vaccinations for Adults with Chronic Liver Disease or Infection This table shows which vaccinations you should have to protect your health if you have chronic hepatitis B or C infection or chronic liver disease (e.g., cirrhosis). Make sure you and your healthcare provider keep your vaccinations up to date. Vaccine Do you need it? Hepatitis A Yes! Your chronic liver disease or infection puts you at risk for serious complications if you get infected with the (HepA) hepatitis A virus. If you’ve never been vaccinated against hepatitis A, you need 2 doses of this vaccine, usually spaced 6–18 months apart. Hepatitis B Yes! If you already have chronic hepatitis B infection, you won’t need hepatitis B vaccine. However, if you have (HepB) hepatitis C or other causes of chronic liver disease, you do need hepatitis B vaccine. The vaccine is given in 2 or 3 doses, depending on the brand. Ask your healthcare provider if you need screening blood tests for hepatitis B. Hib (Haemophilus Maybe. Some adults with certain high-risk conditions, for example, lack of a functioning spleen, need vaccination influenzae type b) with Hib. Talk to your healthcare provider to find out if you need this vaccine. Human Yes! You should get this vaccine if you are age 26 years or younger. Adults age 27 through 45 may also be vacci- papillomavirus nated against HPV after a discussion with their healthcare provider. The vaccine is usually given in 3 doses over a (HPV) 6-month period. Influenza Yes! You need a dose every fall (or winter) for your protection and for the protection of others around you. -
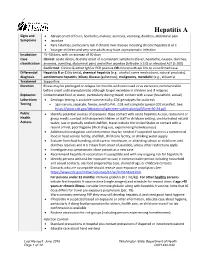
Hepatitis a Reporting Guideline
Hepatitis A Signs and • Abrupt onset of fever, headache, malaise, anorexia, vomiting, diarrhea, abdominal pain Symptoms • Jaundice • Rare fatalities, particularly risk if chronic liver disease including chronic hepatitis B or C • Younger children and very rare adults may have asymptomatic infection Incubation 15–50 days, with an average of 30 days Case Clinical: acute illness, discrete onset of a consistent symptoms (fever, headache, nausea, diarrhea, classification anorexia, vomiting, abdominal pain) and either jaundice (bilirubin ≥ 3.0) or elevated ALT (≥ 200) Confirmed: Clinical & either IgM or PCR positive OR clinical with epi link to a confirmed case Differential Hepatitis B or C (do tests), chemical hepatitis (e.g., alcohol, some medications, natural products), diagnosis autoimmune hepatitis, biliary disease (gallstones), malignancy, metabolic (e.g., Wilson’s) Treatment Supportive Duration Illness may be prolonged or relapse for months with continued virus excretion; communicable before onset until asymptomatic although longer excretion in children and if relapses Exposures Contaminated food or water, particularly during travel; contact with a case (household, sexual) Laboratory • Serologic testing is available commercially; CDC genotypes for outbreak Testing • Spin serum, separate, freeze, send to PHL. CDE will complete special CDC manifest. See: https://www.cdc.gov/laboratory/specimen-submission/pdf/form-50-34.pdf. Public • Identify potential sources of exposure: close contact with acute hepatitis A case, restaurant or Health -

Non-Alcoholic Fatty Liver Disease Information for Patients
April 2021 | www.hepatitis.va.gov Non-Alcoholic Fatty Liver Disease Information for Patients What is Non-Alcoholic Fatty Liver Disease? Losing more than 10% of your body weight can improve liver inflammation and scarring. Make a weight loss plan Non-alcoholic fatty liver disease or NAFLD is when fat is with your provider— and exercise to keep weight off. increased in the liver and there is not a clear cause such as excessive alcohol use. The fat deposits can cause liver damage. Exercise NAFLD is divided into two types: simple fatty liver and non- Start small, with a 5-10 minute brisk walk for example, alcoholic steatohepatitis (NASH). Most people with NAFLD and gradually build up. Aim for 30 minutes of moderate have simple fatty liver, however 25-30% have NASH. With intensity exercise on most days of the week (150 minutes/ NASH, there is inflammation and scarring of the liver. A small week). The MOVE! Program is a free VA program to help number of people will develop significant scarring in their lose weight and keep it off. liver, called cirrhosis. Avoid Alcohol People with NAFLD often have one or more features of Minimize alcohol as much as possible. If you do drink, do metabolic syndrome: obesity, high blood pressure, low HDL not drink more than 1-2 drinks a day. Patients with cirrhosis cholesterol, insulin resistance or diabetes. of the liver should not drink alcohol at all. NAFLD increases the risk for diabetes, cardiovascular disease, Treat high blood sugar and high cholesterol and kidney disease. Ask your provider if you have high blood sugar or high Most people feel fine and have no symptoms. -

COVID-19 and Liver Cirrhosis Important Information for Patients and Their Families
COVID-19 and Liver Cirrhosis Important Information for Patients and Their Families The American Association for the Study of Liver Diseases (AASLD) is committed to helping you understand coronavirus disease 2019 (COVID-19) infection and prevention in people with liver cirrhosis. What We Know Our understanding of COVID-19 in people with liver cirrhosis is evolving. When making decisions related to COVID-19 infections or prevention, having up-to-date information is critical. • Symptoms of COVID-19 infection include any of the following: fever, chills, drowsiness, cough, congestion or runny nose, difficulty breathing, fatigue, body aches, headache, sore throat, abdominal pain, nausea, vomiting, diarrhea, and loss of sense of taste or smell. • People with underlying cirrhosis of the liver are at a higher risk of developing severe COVID-19 illness and/or more problems from their existing liver disease if they get a COVID-19 infection, with prolonged hospitalization and increased mortality. These patients need to take careful precautions to avoid COVID-19 infection. COVID-19 may affect the processes and procedures for screening, diagnosis, and treatment of liver cirrhosis. • Cirrhosis, or scarring of the liver, can be caused by many chronic liver diseases, including viral hepatitis, as well as excessive alcohol intake, obesity, diabetes, diseases of the bile ducts, and a variety of toxic, metabolic, or other inherited diseases. • Most people with liver disease are asymptomatic. Complications, such as yellowing of the skin and eyes from jaundice, internal bleeding (varices), mental confusion (hepatic encephalopathy), and/or swollen belly from ascites, may take years to develop, so patients are often unaware of the severity of their condition and the slow, progressive damage. -

Pancreatic Ascites in a Patient with Cirrhosis and Pancreatic Duct Leak Philip Montemuro, MD Thomas Jefferson University
The Medicine Forum Volume 13 Article 11 2012 Not Your Typical Case Of Ascites: Pancreatic Ascites In A Patient With Cirrhosis And Pancreatic Duct Leak Philip Montemuro, MD Thomas Jefferson University Abhik Roy, MD Thomas Jefferson University Follow this and additional works at: https://jdc.jefferson.edu/tmf Part of the Medicine and Health Sciences Commons Let us know how access to this document benefits ouy Recommended Citation Montemuro, MD, Philip and Roy, MD, Abhik (2012) "Not Your Typical Case Of Ascites: Pancreatic Ascites In A Patient With Cirrhosis And Pancreatic Duct Leak," The Medicine Forum: Vol. 13 , Article 11. DOI: https://doi.org/10.29046/TMF.013.1.012 Available at: https://jdc.jefferson.edu/tmf/vol13/iss1/11 This Article is brought to you for free and open access by the Jefferson Digital Commons. The effeJ rson Digital Commons is a service of Thomas Jefferson University's Center for Teaching and Learning (CTL). The ommonC s is a showcase for Jefferson books and journals, peer-reviewed scholarly publications, unique historical collections from the University archives, and teaching tools. The effeJ rson Digital Commons allows researchers and interested readers anywhere in the world to learn about and keep up to date with Jefferson scholarship. This article has been accepted for inclusion in The eM dicine Forum by an authorized administrator of the Jefferson Digital Commons. For more information, please contact: [email protected]. Montemuro, MD and Roy, MD: Not Your Typical Case Of Ascites: Pancreatic Ascites In A Patient With Cirrhosis And Pancreatic Duct Leak The Medicine Forum Not Your Typical Case Of Ascites: Pancreatic Ascites In A Patient With Cirrhosis And Pancreatic Duct Leak Philip Montemuro, MD and Abhik Roy, MD Case A 55-year-old male with a history of hepatic cirrhosis secondary to Hepatitis C and alcohol abuse presented to an outside hospital with progressive abdominal pain and distension. -

Overview of Causality Assessment for Drug-Induced Liver Injury (DILI) In
Drug Safety https://doi.org/10.1007/s40264-021-01051-5 LEADING ARTICLE Overview of Causality Assessment for Drug‑Induced Liver Injury (DILI) in Clinical Trials Juliana Hey‑Hadavi1 · Daniel Seekins2 · Melissa Palmer3,13 · Denise Cofey2 · John Caminis4 · Sandzhar Abdullaev2 · Meenal Patwardhan5 · Haifa Tyler6 · Ritu Raheja5 · Ann Marie Stanley7 · Liliam Pineda‑Salgado6 · David L. Bourdet8 · Raul J. Andrade9 · Paul H. Hayashi10 · Lara Dimick‑Santos11 · Don C. Rockey12 · Alvin Estilo6 Accepted: 1 February 2021 © The Author(s) 2021 Abstract Causality assessment for suspected drug-induced liver injury (DILI) during drug development and following approval is chal- lenging. The IQ DILI Causality Working Group (CWG), in collaboration with academic and regulatory subject matter experts (SMEs), developed this manuscript with the following objectives: (1) understand and describe current practices; (2) evaluate the utility of new tools/methods/practice guidelines; (3) propose a minimal data set needed to assess causality; (4) defne best practices; and (5) promote a more structured and universal approach to DILI causality assessment for clinical develop- ment. To better understand current practices, the CWG performed a literature review, took a survey of member companies, and collaborated with SMEs. Areas of focus included best practices for causality assessment during clinical development, utility of adjudication committees, and proposals for potential new avenues to improve causality assessment. The survey and literature review provided renewed understanding of the complexity and challenges of DILI causality assessment as well as the use of non-standardized approaches. Potential areas identifed for consistency and standardization included role and membership of adjudication committees, standardized minimum dataset, updated assessment tools, and best practices for liver biopsy and rechallenge in the setting of DILI.