The Genetic and Linguistic Structures of Abyssinians and Their Neighbor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Proposal for Ethiopic Script Root Zone LGR
Proposal for Ethiopic Script Root Zone LGR LGR Version 2 Date: 2017-05-17 Document version:5.2 Authors: Ethiopic Script Generation Panel Contents 1 General Information/ Overview/ Abstract ........................................................................................ 3 2 Script for which the LGR is proposed ................................................................................................ 3 3 Background on Script and Principal Languages Using It .................................................................... 4 3.1 Local Languages Using the Script .............................................................................................. 4 3.2 Geographic Territories of the Language or the Language Map of Ethiopia ................................ 7 4 Overall Development Process and Methodology .............................................................................. 8 4.1 Sources Consulted to Determine the Repertoire....................................................................... 8 4.2 Team Composition and Diversity .............................................................................................. 9 4.3 Analysis of Code Point Repertoire .......................................................................................... 10 4.4 Analysis of Code Point Variants .............................................................................................. 11 5 Repertoire .................................................................................................................................... -

Traditional Medicinal Plants Used by Kunama Ethnic Group in Northern Ethiopia
Vol. 9(15), pp. 494-509, 17 April, 2015 DOI: 10.5897/JMPR2014.5681 Article Number: 8DFCBAC52764 ISSN 1996-0875 Journal of Medicinal Plants Research Copyright © 2015 Author(s) retain the copyright of this article http://www.academicjournals.org/JMPR Full Length Research Paper Traditional medicinal plants used by Kunama ethnic group in Northern Ethiopia Meaza Gidey1*, Tadesse Beyene2, Maria Adele Signorini3, Piero Bruschi4 and Gidey Yirga2 1Department of Biology, Aksum University P.O. Box 287 Tigray, Ethiopia. 2Department of Biology, Mekelle University, PO Box 231 Tigray, Ethiopia. 3Department of Biology, University of Florence PO Box 100547, Florence, Italy. 4Department of Agriculture, Food and Environmental Science, sect. soil and Plant science, University of Florence, p.le Cascine 28 Firenze, Italy. Received 7 November, 2014; Accepted 17 April, 2015 Utilization of medicinal plants is almost as old as the history of mankind. Semi-structured interview, guided field walk, group discussion and market survey were used to collect ethnobotanical data in Tahitay Adiyabo and Kafta Humera districts in northern Ethiopia. A total of 47 informants (30 males and 17 females) were selected purposefully from three sub-districts: Lemlem (n = 27), Adi-Goshu (n = 10) and Hilet-Coca (n = 10). A total of 115 species of medicinal plants were collected and identified for treating 59 humans and livestock ailments. The most commonly used plant parts for herbal preparations were roots (35.5%) and leaves (21.74%) and were administered through oral, dermal, ocular, nasal and vaginal routes in decreasing order. Oral application (58 preparations, 50.43%) was the highest and most commonly used route of application followed by dermal application (35 preparations, 30.43%). -

Similative Morphemes As Purpose Clause Markers in Ethiopia and Beyond Yvonne Treis
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Archive Ouverte a LUniversite Lyon 2 Similative morphemes as purpose clause markers in Ethiopia and beyond Yvonne Treis To cite this version: Yvonne Treis. Similative morphemes as purpose clause markers in Ethiopia and beyond. 2016. <hal-01351924> HAL Id: hal-01351924 https://hal.archives-ouvertes.fr/hal-01351924 Submitted on 4 Aug 2016 HAL is a multi-disciplinary open access L'archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destin´eeau d´ep^otet `ala diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publi´esou non, lished or not. The documents may come from ´emanant des ´etablissements d'enseignement et de teaching and research institutions in France or recherche fran¸caisou ´etrangers,des laboratoires abroad, or from public or private research centers. publics ou priv´es. Similative morphemes as purpose clause markers in Ethiopia and beyond Yvonne Treis LLACAN (CNRS, INALCO, Université Sorbonne Paris-Cité) Abstract In more than 30 languages spoken at the Horn of Africa, a similative morpheme ‘like’ or a noun ‘manner’ or ‘type’ is used as a marker of purpose clauses. The paper first elaborates on the many functions of the enclitic morpheme =g ‘manner’ in Kambaata (Highland East Cushitic), which is used, among others, as a marker of the standard in similative and equative comparison (‘like’, ‘as’), of temporal clauses of immediate anteriority (‘as soon as’), of complement clauses (‘that’) and, most notably, of purpose clauses (‘in order to’). -

App-1. Propagation of Language 173 Appendix 1. Propagation Of
App-1. Propagation of language 173 Appendix 1. Propagation of language- the languages of Ethiopia This piece has 12 parts. It describes the propagation of language, gives the names of most languages of Ethiopia, with emphasis on Amarigna, Oromigna, Somaligna, and Tigrigna. It also indicates difficulties of organizing Ethiopians on a federal structure composed of language- based regions. 1. Language does not indicate which group of people ruled over other groups, nor does it change the ethnicity or origin of people. A language (superstratum) propagates over another (substratum). Consider the following English phrase as a way of examining a superstratum and substratum. [1] Superstratum: "the southern people"-- as spoken by an. Englishman Substratum-a: "ze saz'n pipl"-- as spoken by a non- Englishman. Substartum-b: "za souzern bibl"--as spoken by another non-Englishman. The same language (superstratum) propagating on different languages (substrata) may result in separate dialects, or even different languages if the substrata are different from each other. For example, different Roman legions (speaking Latin) ruled over Western Europe that App-1. Propagation of language 174 resulted in the Spanish, Portuguese, and French languages. The propagation of a language does not change the origin of people, as the Portuguese are not Italians or Frenchmen though their speech is rooted in Latin (superstartum). This would be an example of how the language of the rulers propagated on the subjects. Yet, there are cases where the language of the subjects propagated on the rulers. An example of the latter case would be the Manchurians that invaded China and ruled over them for centuries. -

Ethnic Group and Mother Tongue in the Ethiopian Censuses of 1994 and 2007
Ethnic Group and Mother Tongue in the Ethiopian Censuses of 1994 and 2007 GROVER HUDSON, Michigan State University, East Lansing The Ethiopian census of 2007 (POPULATION CENSUS COMMISSION 2007) provides information on ethnic groups (Table 3.1, pp. 98–122) and mother tongues (Table 3.2, pp. 123–148), as did the census of 1994 (POPULATION CENSUS COMMISSION 1994; HUDSON 2003; 2004). We read (POPULATION CENSUS COMMISSION 2007: 96) that “Detailed lists of the names & codes of the country’s languages were provided to the enumerators in their instruction manuals. Similar types of questions were used for both the 1994 & 2007 cen- suses, except that during the 1994 census [a] question about the most fre- quently used additional language of respondents was also asked in addition to mother tongue & ethnic group”. Presented here in four tables are comparisons of the “country total” cen- sus results for 2007 and 1994 concerning ethnic group and mother tongue of respondents, with attention to differences between the two censuses and within each census between ethnic groups and mother tongues (ethnic groups without mother tongues and vice versa). Some of the differences can be explained with reasonable confidence but some only as suppositions; some remain unexplained. (The census also provides results by Regions: Tǝgray; ʿAfar; Amhara; Oromia; Somali; Benišangul-Gumuz; Southern Nations, Nationalities, and Peoples (SNNP); Gambella; Harari; Addis Abäba; Dǝrre Dawa; and (sic) “Region 17”.) The 2007 census reported two ethnic groups without associated mother tongues (Mejenger, Silte; s. notes 27, 34), and 5 mother tongues without an associated ethnic group (Demegna, Felashigna, Koyrigna, Shetagna, Shitagna; s. -

Documentation and Description of the Ethnobotany and Ethnozoology of the Zay Ethnolinguistic Group
ADDIS ABABA UNIVERSITY SCHOOL OF GRADUATE STUDIES Documentation and Description of the Ethnobotany and Ethnozoology of the Zay Ethnolinguistic Group By: Fekadu Beshah May, 2014 Addis Ababa, Ethiopia 1 Documentation and Description of the Ethnobotany and Ethnozoology of the Zay Ethnolinguistic Group A thesis submitted in partial fulfillment of the requirements for the Degree of Master of Arts in Documentary Linguistics and Culture Advisor: Zelealem Leyew, PhD 2 3 ADDIS ABABA UNIVERSITY The undersigned hereby certify that they have read and recommend for acceptance of a thesis entitled “Documentation and Description of Ethnobotany and Ethnozoology of Zay Ethnolinguistic Group” by Fekadu Beshah in partial fulfillment of the requirements for the degree of Master of Arts in Documentary Linguistics and Culture. Examiner _____________________Signature _______________Date_______________ Examiner _____________________Signature _______________Date________________ Advisor ______________________ Signature _______________Date________________ _________________________________________________ Chairman, Department or Graduate coordinator 4 5 Abstract The Zay people are known for their interaction with plants and animals in which their traditional knowledge, culture and language are expressed. However, despite the existence of rich indigenous practices related to the ethnobotany and ethnozoology of this ethnolinguistic group, this people remain unexplored and no comprehensive account of their plant and animal utilization is available. In addition, due to lack of sufficient land for residence and farming, many members of the Zay community are migrating from their area to the nearby towns (Endashaw 2010:4). Therefore, the situation calls for an urgent action to collect and document their indigenous knowledge and practices including those plants and animals. Hence, the aim of this study is to describe and document the ethnobotany and ethnozoology of the Zay ethnolinguistic group. -

Programme of the Conference
PROGRAMME 19th International Conference of Ethiopian Studies Ethiopia - Diversity and Interconnections through Space and Time Warsaw, 24-28 August 2015 www.ices19.uw.edu.pl Plan of University of Warsaw Campus OLD LIBRARY BUILDING (STARY BUW) FACULTY OF ORIENTAL STUDIES FACULTY OF HISTORY & SALA KOLUMNOWA 2 19th International Conference of Ethiopian Studies, Warsaw 24-28 August 2015 Plan of University of Warsaw Campus Towards Vistula River Stary BUW Faculty of Oriental Studies Faculty of History & Sala Kolumnowa Krakowskie Przedmieście & Main Gate Towards State Towards National Museum Ethnographical Museum 3 19th International Conference of Ethiopian Studies, Warsaw 24-28 August 2015 Plan of Old Library Building (Stary BUW) Ground floor ENTRANCE REGISTRATION & EXHIBITION HALL AULA 4 19th International Conference of Ethiopian Studies, Warsaw 24-28 August 2015 Plan of Old Library Building (Stary BUW) first floor 106 108 109 107 105 116 111 115 112 114 113 5 19th International Conference of Ethiopian Studies, Warsaw 24-28 August 2015 VENUES UNIVERSITY OF WARSAW, KRAKOWSKIE PRZEDMIEŚCIE 24/26 OLD LIBRARY BUILDING (STARY BUW) Ground floor: Aula First floor: rooms 105, 106, 107, 111, 112, 113, 114, 115, 116 FACULTY OF ORIENTAL STUDIES First floor: Schayer Room (Sala Schayera) Second floor: rooms 208, 209 FACULTY OF HISTORY Ground Floor: Sala Kolumnowa NATIONAL MUSEUM IN WARSAW, AL. JEROZOLIMSKIE 3 (MNW) 6 19th International Conference of Ethiopian Studies, Warsaw 24-28 August 2015 LIST OF PANELS TITLE DAY AND HOUR ROOM GENERAL PANELS I Law and -

A Sociolinguistic Survey Report of the Zay People in Ethiopia
DigitalResources Electronic Survey Report 2011-046 ® A Sociolinguistic Survey Report of the Zay People in Ethiopia Linda Jordan Jillian Netzley Hussein Mohammed A Sociolinguistic Survey Report of the Zay People in Ethiopia Linda Jordan, Jillian Netzley, and Hussein Mohammed SIL International® 2011 SIL Electronic Survey Report 2011-046, November 2011 © 2011 Keith Beavon, Ann Elizabeth Johnson, and SIL International® All rights reserved 2 Abstract 1. Introduction 1.1 Geography 1.2 People and language 1.3 Other previous research 2. Goals of the research 3. Methodology 3.1 Procedures 3.2 Data sources 3.3 Analysis 4. Results 4.1 Sociolinguistic questionnaires 4.2 Community ownership and support questionnaire 4.3 Oromo SRT 4.4 Amharic RTT 5. Data analysis 5.1 Sociolinguistic questionnaire 5.2 Community ownership and support questionnaire 5.3 Mean SRT scores for men and women 5.4 Mean RTT scores for men and women 6. Conclusions and recommendations Appendix A. Sociolinguistic questionnaire Appendix B. B.1. Descriptive statistics - SRT B.2 Two sample T-test and confidence interval B.3 Descriptive statistics - RTT B.4 Two sample T-test and confidence interval References 3 Abstract The optimal language for literature and educational materials is not the same for all Zay areas. The data gathered during the current study points to Zay as optimal for the islands on Lake Ziway and Oromo as optimal for the lakeshores. However, the Zay people living on the islands would probably be well served by Amharic literature and educational materials until most of them immigrate to the shore or the Oromo educational system causes a shift in preference to Oromo. -
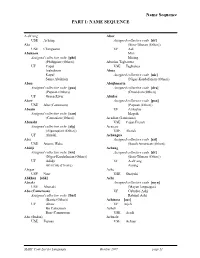
PART I: NAME SEQUENCE Name Sequence
Name Sequence PART I: NAME SEQUENCE A-ch‘ang Abor USE Achang Assigned collective code [sit] Aba (Sino-Tibetan (Other)) USE Chiriguano UF Adi Abaknon Miri Assigned collective code [phi] Miśing (Philippine (Other)) Aborlan Tagbanwa UF Capul USE Tagbanua Inabaknon Abua Kapul Assigned collective code [nic] Sama Abaknon (Niger-Kordofanian (Other)) Abau Abujhmaria Assigned collective code [paa] Assigned collective code [dra] (Papuan (Other)) (Dravidian (Other)) UF Green River Abulas Abaw Assigned collective code [paa] USE Abo (Cameroon) (Papuan (Other)) Abazin UF Ambulas Assigned collective code [cau] Maprik (Caucasian (Other)) Acadian (Louisiana) Abenaki USE Cajun French Assigned collective code [alg] Acateco (Algonquian (Other)) USE Akatek UF Abnaki Achangua Abia Assigned collective code [sai] USE Aneme Wake (South American (Other)) Abidji Achang Assigned collective code [nic] Assigned collective code [sit] (Niger-Kordofanian (Other)) (Sino-Tibetan (Other)) UF Adidji UF A-ch‘ang Ari (Côte d'Ivoire) Atsang Abigar Ache USE Nuer USE Guayaki Abkhaz [abk] Achi Abnaki Assigned collective code [myn] USE Abenaki (Mayan languages) Abo (Cameroon) UF Cubulco Achi Assigned collective code [bnt] Rabinal Achi (Bantu (Other)) Achinese [ace] UF Abaw UF Atjeh Bo Cameroon Acholi Bon (Cameroon) USE Acoli Abo (Sudan) Achuale USE Toposa USE Achuar MARC Code List for Languages October 2007 page 11 Name Sequence Achuar Afar [aar] Assigned collective code [sai] UF Adaiel (South American Indian Danakil (Other)) Afenmai UF Achuale USE Etsako Achuara Jivaro Afghan -

Population Status of Five Hawaiian Endemic Fern Taxa Within the Genus Diellia (Aspleniaceae)
Aguraiuja: Population status of five Hawaiian endemic fern taxa within the genus Diellia (Aspleniaceae) CBM:s Skriftserie 3: 724 Uppsala 2001 Population status of five Hawaiian endemic fern taxa within the genus Diellia (Aspleniaceae) Ruth Aguraiuja Aguraiuja, R$ 2001$ Population status of five Hawaiian endemic fern taxa within the genus Diellia (Aspleniaceae)$ CBM:s Skriftserie 3: 724$ The life-stage and size structure of 10 populations of five endemic fern taxa was studied (Diellia erecta Brack; D$ falcata Brack; D$ pallida WH Wagner; D$ unisora WH Wagner and D x lauii (D$ falcata x D$ unisora) WH Wagner), with the aim of assessing the overall condition of the populations During the study, populations were defined as spatially distinct assemblages of plants at certain sites, with no consideration of the genetic structure of the populations Spatial patchiness of habitat, clumped distribution patterns, life-stage specific survival and slow establishment of gametophytes are characteristic of all taxa of the genus Diellia Data from field observations of D$ falcata over three months lead to the assumption that Diellia ferns show a continuous growth pattern with irregular periods of dormancy and spore release with defined peaks The proportion of vegetative premature and reproductively mature sporophytes could be one possible variable for use in the assessment of the population status of Diellia ferns in short-term studies The single population of D$ pallida in Mahanaloa Valley, Kauai, is in critical condition with only 31 individu- als of -

Bulletin of the Department of Linguistics & Philology
Bulletin of the Department of Linguistics & Philology College of Humanities, Language Studies, Journalism and Communication Addis Ababa University P.O.Box 1176 Tel. (251-1) 23 97 55 Email: [email protected] Bulletin of the Department of Linguistics & Philology In this Issue Editorial 4 Editorial Committee News 6 Shimelis Mazengia • AAU and HU Conduct Linguistic Capacity Building Workshops 6 Abebayehu Messele • Seven Staff Members Graduate with Doctoral Degrees. 9 Beniam Mitiku • Encyclopaedia Aethiopica Completed 11 Dawit Hassen (Layout) • A Dept. Contingent Participates in a Conference at Hamburg Mihiret Daba (Cover) University 11 • Preparation of The Oxford Handbook of Ethiopian Languages in Progress ...............................................................................................12 • Training Given in Ethiopian Sign Language 14 • Staff Participate in Various Academic Activities 15 • Talks and Lectures Given and Workshops Conducted by Guest Scholars 18 • Symposium Held in Memory of Prof. Marrassini at AAU 24 Synopses of Doctoral Dissertations 25 • Clinical Linguistics 25 • Experimental Phonetics 29 • Theoretical and Descriptive Linguistics 39 • Philology 45 Enrollment (2007 E.C.) 54 Recent Publications of Staff (2013-2014) 55 College of Humanities, Language Studies, Journalism and Communication Staff Profile (2014) 57 Addis Ababa University Bulletin of the Department of Linguistics & Philology NO 6 Bulletin of the Department of Linguistics & Philology NO 6 Editorial bulletin bears a new title—Bulletin of the Department of Linguis- tics & Philology. The new title is believed to be more transparent From DLing to Bulletin of the Department of Linguistics & Philology and inclusive as well as reflective of the mission of the department. DLing, the bulletin of the Department of Linguistics and Phi- Apart from presenting news and reports related to the activi- lology, was launched in 2005 and came out in five issues till ties of the department, as did the previous issues, this issue addi- 2009. -

Downloaded From
The struggle for recognition: a critical ethnographic study of the Zay Vinson, M.A. Citation Vinson, M. A. (2012). The struggle for recognition: a critical ethnographic study of the Zay. Retrieved from https://hdl.handle.net/1887/24136 Version: Not Applicable (or Unknown) License: Downloaded from: https://hdl.handle.net/1887/24136 Note: To cite this publication please use the final published version (if applicable). THE STRUGGLE FOR RECOGNITION A CRITICAL ETHNOGRAPHIC STUDY OF THE ZAY Michael A. Vinson African Studies Centre Universiteit Leiden Supervisors: J. Abbink & S. Luning Table of Contents List of Zay Terms ............................................................................................................... iv List of Tables ....................................................................................................................... v List of Figures ..................................................................................................................... v Acknowledgements ............................................................................................................ vi 1 Introduction .......................................................................................................... 7 1.1 Background and Rationale ........................................................................................ 8 1.2 Problem Statement and Research Questions ......................................................... 11 1.3 Methods ..................................................................................................................