CHAPTER 14 Models of Feed Utilization and Growth for Monogastric Animals V
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Herbivore Digestive System Buffalo Zebra
The Herbivore Digestive System Name__________________________ Buffalo Ruminant: The purpose of the digestion system is to ______________________________ _____________________________. Bacteria help because they can digest __________________, a sugar found in the cell walls of________________. Zebra Non- Ruminant: What is the name for the largest section of Organ Color Key a ruminant’s Mouth stomach? Esophagus __________ Stomach Small Intestine Cecum Large Intestine Background Information for the Teacher Two Strategies of Digestion in Hoofed Mammals Ruminant Non‐ruminant Representative species Buffalo, cows, sheep, goats, antelope, camels, Zebra, pigs, horses, asses, hippopotamus, rhinoceros giraffes, deer Does the animal Yes, regurgitation No regurgitation regurgitate its cud to Grass is better prepared for digestion, as grinding Bacteria can not completely digest cell walls as chew material again? motion forms small particles fit for bacteria. material passes quickly through, so stool is fibrous. Where in the system do At the beginning, in the rumen Near the end, in the cecum you find the bacteria This first chamber of its four‐part stomach is In this sac between the two intestines, bacteria digest that digest cellulose? large, and serves to store food between plant material, the products of which pass to the rumination and as site of digestion by bacteria. bloodstream. How would you Higher Nutrition Lower Nutrition compare the nutrition Reaps benefits of immediately absorbing the The digestive products made by the bacteria are obtained via digestion? products of bacterial digestion, such as sugars produced nearer the end of the line, after the small and vitamins, via the small intestine. intestine, the classic organ of nutrient absorption. -

Ruminant Animal? Many Different Species of Ruminant Animals Are Found Around the World
What is a Ruminant Animal? Many different species of ruminant animals are found around the world. Ruminants include cattle, sheep, goats, buffalo, deer, elk, giraffes and camels. These animals all have a digestive system that is uniquely different from our own. Instead of one compartment to the stomach they have four. Of the four compartments the rumen is the largest section and the main digestive centre. The rumen is filled with billions of tiny microorganisms that are able to break down grass and other coarse vegetation that animals with one stomach (including humans, chickens and pigs) cannot digest. Ruminant animals do not completely chew the grass or vegetation they eat. The partially chewed grass goes into the large rumen where it is stored and broken down into balls of “cud”. When the animal has eaten its fill it will rest and “chew its cud”. The cud is then swallowed once again where it will pass into the next three compartments—the reticulum, the omasum and the true stomach, the abomasum. Dairy calves have a four-part stomach when they are born. However, they function primarily as a monogastric (simple-stomached) animal during the first part of their lives. At birth the first three compartments of a calf’s stomach—rumen, reticulum, and omasum—are inactive and undeveloped. As the calf grows and begins to eat a variety of feeds, its stomach compartments also begin to grow and change. The abomasum constitutes nearly 60 percent of the young calf’s stomach, decreasing to about 8 percent in the mature cow. The rumen comprises about 25 percent of the young calf’s stomach, increasing to 80 percent in the mature cow. -

Biogas Production from Ruminant and Monogastric Animal Manure Co-Digested with Manipueira
Biogas production from ruminant and monogastric animal manure co-digested with manipueira Andrade, W.R.1@; Xavier, C.A.N.2; Coca, F.O.C.G.2; Arruda, L.D.O.1 and Santos, T.M.B.2 1Animal Science. State University of Mato Grosso do Sul. Aquidauana. MS. Brazil. 2Animal Science. Federal University of Mato Grosso do Sul. Campo Grande. MS. Brazil. SUMMARY ADDITIONAL KEYWORDS The aim of this study was to evaluate ruminant and monogastric animal manure co-digested Alkalinity. with 10 % of manipueira through process monitoring parameters and biogas production. In this Ammonia. study, eight semi-continuous digesters, with a capacity of 7.8 liters of substrate in fermentation, Digester. operated with 30 days of hydraulic retention time, were used. Monitoring analyses were per- Cassava. formed in order to assess: pH, total ammonia nitrogen (TAN) concentration, partial alkalinity 3 -1 pH. (PA), content and biogas yield (m ·kg·Volatile Solids (VS)added ). There was no statistic difference for all the pH values, which reached values around 6.8 to 8.0. For PA, all the substrates rea- ched higher than 1.200 mg·L-1 values as recommended. During the process, there was no risk of failure due to TAN concentration. The average biogas productions accumulated per week were 0.00676; 0.01167; 0.01515 and 0.01856 m3 for substrates composed by dairy cattle, sheep, poultry and swine manure co-digested with manipueira, respectively. The biogas yield 3 -1 for those respective substrates were 0.122; 0.275; 0.535 e 0.843 m ·kg·VSadded . -

Nutrition Digestive Systems
4-H Animal Science Lesson Plan Nutrition Level 2, 3 www.uidaho.edu/extension/4h Digestive Systems Sarah D. Baker, Extension Educator Goal (learning objective) Pre-lesson preparation Youth will learn about the differences, parts and Purchase supplies (bread, soda, orange juice, functions between ruminant and monogastric diges- Ziploc baggies) tive systems. Make copies of Handouts 1, 2, and 3 for group Supplies Prepare bread slices Copies of Handout 1 “Ruminant vs Monogastric Make arrangements to do the meeting in a lo- Digestive System” make enough copies for group cation that has internet connection, tables, and Copies of Handout 2 “Ruminant Digestive System chairs – Parts and Functions” make enough copies for Read/review lesson group Watch video Copies of Handout 3 “Monogastric Digestive Sys- Test computer/internet connection and video be- tem – Parts and Functions” make enough copies fore meeting https://youtu.be/JSlZjgpF_7g for group Computer (may need speakers depending on facil- Lesson directions and outline ity and group size) Share the following information with the youth: Internet connection to view YouTube video The definition of digestion is the process of break- Slices of bread cut into 4 squares (each member ing down food by mechanical and enzymatic action in will need one square of bread) the stomach and intestines into substances that can be used by the body. The digestive system performs five Sandwich size Ziploc baggies (one bag for each major functions: member) 1. Food intake One, three-ounce cup for holding liquid (one cup for each member) 2. Storage 1 Liter of bottle of soda 3. -

Comparison of Digestive Systems Introduction Organisms Depend On
Comparison of Digestive Systems Introduction Organisms depend on nutrients to maintain metabolic needs. All animals are consumers (a.k.a. heterotrophs), meaning they do not have the ability to manufacture their own food; therefore, they must consume other organisms as food. The primary purpose of the digestive system is to break down the consumed foods and transfer nutritional components to the body’s cells for use in production of the body’s fuel and ATP as well as to manufacture other biomolecules. There are three categories animals can be placed into based on their diets: herbivore, carnivore, and omnivore. Herbivores consume plant matter, whereas the primary diet of a carnivore is other animals. Omnivores consume both plant and animal matter. In vertebrates, the structures that vary between organisms based on diet often include differences in dentition and gut length. Herbivore Herbivores consume algae or plant matter such as seeds, leaves, and fruits. Because some of these materials are low in easily accessible energy, herbivores have evolved two alternatives for releasing the nutrients: foregut and hindgut gastric fermentation. Gastric fermentation utilizes bacteria that breakdown the hard to digest cellulose, the plant cell wall’s primary component. In animals that use foregut (a.k.a. pre-gastric) fermentation, the stomach is modified into four chambers/compartments where the first chamber is the rumen. This rumen provides a place for the bacterial breakdown of food. Ruminants regurgitate the partially digested mass from their rumen, known as “cud,” and continue to chew the plant matter to break it down further. Examples of foregut ruminants include cows, sheep, camels, and deer. -

Listeriosis Caused by the Bacterium Listeria Monocytogenes
PUBLIC HEALTH WATCH V-1226 FOCUS ON AGRICULTURE Listeriosis caused by the bacterium Listeria monocytogenes Neil W. Dyer The disease in humans The disease in ruminants DVM, DACVP • Opportunistic infection of: (cattle, sheep and goats) Director, NDSU Diagnostic Laboratory – Elderly • Encephalitis (infection of the Charles L. Stoltenow – Pregnant women central nervous system). DVM, DACVPM – Unborn – “Circling disease” – caused by Extension Veterinarian brain involvement – Neonates – Immunosuppressed • Abortion (infection of the uterus Listeriosis is an infection of human and or the fetus). animal importance. It is caused by • Pregnant women may experience a – Generally during the last two Listeria monocytogenes, a bacterium mild infection (flu-like symptoms) months of gestation because widely distributed in the environment. which can spread to the fetus and of production practices In particular it can be found in soil, lead to intrauterine death or a water, a variety of foodstuffs, and feces of severe neonatal infection. – Occurs in winter healthy animals and people. It is capable – Infections early in pregnancy are – Associated with consumption of causing disease in humans, animals treatable of spoiled silage or spoiled hay and birds. The bacterium can survive – Infections in late pregnancy – Animals that abort are resistant in soil or silage for up to two years. may cause death of the fetus or to reinfection It is capable of growing in a wide variety problems in the development of foods under a number of different • Septicemia (infection of the blood). of the nervous system environmental conditions such as high – Primarily occurs in young – Surviving infants show salt concentration, acid environment, animals long-term problems and refrigerator temperatures. -
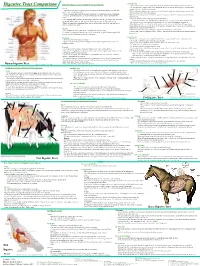
Digestive Tract Comparison • the Small Intestine Is a Tube Roughly Twenty Feet Long Deided Into the Duodenum, Jejunum and Ileum
• Small Intestine Human/Dog Digestive system or Simple Monogastric Digestion Digestive Tract Comparison • The small intestine is a tube roughly twenty feet long deided into the duodenum, jejunum and ileum. • The first part of the small intestine is the duodenum, the site of most chemical digestive reactions and is Mouth smoother than the rest of the small intestine • A specialized region of the digestive tract designed to break up large particles of food into • Bile, bicarbonate and pancreatic enzymes are secreted into the duodenum to breakdown nutrients in the smaller, more manageable particles chyme so that they can be readily absorbed. • Saliva is added to moisten food and begin carbohydrate breakdown by amylase in humans. •Bicarbonate from the pancreas neutralizes corrosive stomach acid from 3.5 in the stomach to 8.5 in the • There are four main types of teeth in the human or dog: incisors, canines, premolars and small intestine. molars. •Pancreatic enzymes include lipases, peptidases and amylases. •One reason dog and cat canines are much larger than ours is that they need to be able to rip and •Lipases break down fats. Peptidases break down proteins. Amylases break down carbohydrates. tear through tough raw meat. Humans have evolved to eat easier to chew, cooked meat. • Bile from the liver is stored in the gall bladder and secreted into the duodenum to emulsify fat. • While chewing, food is transformed into what is called a bolus, a food ball, and then forced •The jejunum and ileum are next in the small intestine and are covered in villi, finger-like projections. -

0 and Digestive Physiology in Monogastric Farm Animals
Nutrition ^ v; 0 anddigestiv e physiology inmonogastri c farmanimal s Reviews presented at the symposium on the occasion of the retirement of Dr Ir E.J. van Weerden, Wageningen, Netherlands, 26 May 1989 E.J. van Weerden & J. Huisman (Editors) Pudoc Wageningen 1989 !-"' i~* \ T>Y\ CIP data, Royal Netherlands Library, The Hague Nutrition anddigestiv e physiology in monogastric farm animals. Reviews presented at the symposium onth e occasiono f the retirement of Dr Ir E.J.va nWeerden ,Wageningen , Netherlands, 26 May 1989. / E.J. van Weerden &J. Huisman (Editors). - Wageningen: Pudoc. - x+IOI pp. ISBN 90-220-1011-2 UDC 636.2/.5.084:59l.l32 NUGI 835 Subject heading: animal nutrition © Centre for Agricultural Publishing & Documentation (Pudoc), Wageningen, Netherlands, 1989 All rightsreserved .Nothin gfro m thispublicatio n mayb ereproduced ,store d ina computerize d system or published in any form or in any manner, including electronic, mechanical, reprographic or photo graphic, without prior written permission from the publisher, Pudoc, P.O. Box 4, 6700 AA Wage ningen, Netherlands. The individualcontribution s inthi spublicatio n andan yliabilitie s arisingfro m them remainth e responsi bility of the authors. Insofar as photocopies from thispublicatio n arepermitte d byth e Copyright Act 1912,Articl e I6Ban d Royal Netherlands Decree of 20Jun e 1974(Staatsbla d 351) asamende d in Royal Netherlands Decree of 23Augus t 1985(Staatsbla d47 1) an db yCopyrigh t Act 1912,Articl e 17,th e legallydefine d copyright fee for anycopie sshoul db etransferre d to the StichtingReprorech t (P.O.Bo x 882, 1180 AW Amstel veen, Netherlands). -

Listeriosis in Fattening Pigs Caused by Poor Quality Silage
Stein et al. BMC Veterinary Research (2018) 14:362 https://doi.org/10.1186/s12917-018-1687-6 CASEREPORT Open Access Listeriosis in fattening pigs caused by poor quality silage - a case report Heiko Stein1,5, Beatrix Stessl2, Rene Brunthaler3, Igor Loncaric4, Herbert Weissenböck3, Ursula Ruczizka1, Andrea Ladinig1 and Lukas Schwarz1* Abstract Background: Listeria (L.) monocytogenes as the causative agent of listeriosis in humans and different animal species, has its reservoir in the environment. It can be found in the gut and faeces of healthy pigs, but under certain circumstances it may cause clinical disease. Fatteners are usually not known to get affected by Listeria-associated septicaemia and enteritis. This case report shows, that L. monocytogenes should be part of the list of differential diagnoses, when fattening pigs suffer from haemorrhagic diarrhoea and septicaemia. Case presentation: Here, we report of an episode of fatal listeriosis in fattening pigs in a piglet producing farm in Lower Austria, which was combined with a fattening unit with space for 450 fatteners. The mortality rate resulted in 7.8% among fattening pigs after suffering from clinical symptoms such as anorexia, bloody diarrhoea and increased body temperature. Two fattening pigs with clinical symptoms and maize silage samples were used for further diagnostics. L. monocytogenes were isolated from serosa samples of the pigs and in the corresponding fed maize silage. One animal was positively tested for Brachyspira hyodysenteriae, which may have also been involved in the development of colitis. Immunohistochemically, L. monocytogenes could be detected in high amounts in lymphatic tissue of the gut. Molecular biological characterisation of the L. -

Animal Science Anywhere, a Stomach at Work
4H1659 4ANIMAL DIGESTION ACTIVITY A Stomach At Work Skill Level: Overview: 4 Beginner to intermediate The Animal Digestion – A Stomach At Work lesson is designed to teach young people about animal digestion. Participants will complete an Life Skills: activity in which they “digest” a slice of bread the way their stomachs 4 Critical thinking, decision-making and would. They’ll also discuss the basic differences in how various species problem-solving of livestock digest feed. In the interactive lesson, they’ll also learn the Setting: importance of acids to the process of digestion. 4 An outdoor or indoor space with a Objectives: supply station and an easy to clean After completing this activity, participants will be able to: floor; seating is optional. 4 Discuss how digestive acids break down carbohydrates. Time: 4 Explain the difference between monogastric and ruminant animals. 4 20–30 minutes Materials: ❏ Resealable plastic sandwich bags PROCEDURE: (one per participant plus one for Before the meeting: demonstration) 1. Review the lesson and gather any supplies you will need. Make one ❏ Sliced white bread (one piece per copy of “The Basics of the Ruminant Digestive System” resource participant plus one for demonstration) sheet per person (optional). ❏ 2-liter bottles of orange juice or cola (about one bottle per 15 participants) 2. Place one piece of white bread in a resealable plastic sandwich ❏ 3-ounce disposable cups (one per bag and fill one 3-ounce cup with orange juice or cola for each participant) participant. Set the sandwich bags and filled cups out on the ❏ Paper towels (one sheet per participant supply station. -

The Digestive System (Gastrointestinal Tract) (Alimentary
VET-114 Animal Anatomy and Physiology 2 Lesson 3 Digestive and Endocrine Systems Chapters 11, 12, 15 The Digestive System Chapter 11 Pages 264-282 Textbook Learning Objectives Chapter 11 – Page 264 • List the functions of the digestive system. • Describe the epithelial and muscle layers of the gastrointestinal tract. • Explain the process of peristalsis. • List the structures of the oral cavity. • List and give the locations of the salivary glands. • Name the types of teeth found in carnivores and herbivores and describe the structure of teeth. • Differentiate between mechanical and chemical digestion. • List the structures that make up the monogastric stomach and describe the function of each area. • Explain the effect(s) on the gastrointestinal system of amylase, lipase, gastrin, pepsin, pepsinogen, prostaglandins, mucin, bicarbonate, secretin, cholecystokinin, proteases, and hydrogen and chloride ions. • Describe the structure and functions of the rumen, reticulum, omasum, and abomasum. • Differentiate between fermentative and nonfermentative digestion. • List the segments of the small and large intestine and describe the general functions of each segment. The Digestive System • Gastrointestinal tract (GIT) • Alimentary canal Putting Things in Perspective! VERY important system clinically! The “Tracts” of Internal Medicine The Digestive System (GIT) Figure 11-1, Page 265 • Digestive tract, gastrointestinal (GI) tract, alimentary canal, gut • Tube that runs from the mouth to the anus; accessory digestive organs Basic Functions of GIT Digestion of Macronutrients Absorption of All Nutrients Elimination of Wastes Digestion of Macronutrients • Definition – large nutrient molecules that require breakdown into smaller molecules before being absorbed . “Energy” nutrients (Calories) . Carbohydrates . Fats . Proteins Digestion of Macronutrients Absorption of All Nutrients • Macronutrients • Micronutrients – nutrient molecules so small that no digestion is required before being absorbed . -
Course Agricultural Science I Unit Introduction to Animal Nutrition
Course Agricultural Science I Unit Introduction to Animal Nutrition Lesson Monogastric Digestive System Estimated Time 50 minutes Student Outcome Identify the components and describe the functions of the monogastric animal digestive system. Learning Objectives 1. Determine which domestic animals have monogastric digestive systems. 2. Identify the components of the monogastric digestive system. 3. Describe the functions that the components of the monogastric system play in digestion. 4. Explain the role of digestive juices and enzymes in the digestion of nutrients. Grade Level Expectations SC/LO/2/D/09-11/c SC/LO/2/F/09-11/a SC/LO/2/D/09-11/d SC/LO/2/F/09-11/d Resources, Supplies & Equipment, and Supplemental Information Resources 1. PowerPoint Slides PPt 1 – Swine Digestive System PPt 2 – Horse Digestive System PPt 3 – Dog Digestive System PPt 4 – Rabbit Digestive System PPt 5 – Fowl Digestive System - Chicken PPt 6 – Enzymes in the Monogastric Digestive System 2. Activity Sheets AS 1 – Swine Digestive System AS 2 – Dissection of the Monogastric Digestive System 3. Introduction to Animal Nutrition (Student Reference). University of Missouri-Columbia: Instructional Materials Laboratory, 1996. 4. Introduction to Animal Nutrition Curriculum Enhancement. University of Missouri- Columbia: Instructional Materials Laboratory, 2003. Supplies & Equipment A monogastric digestive system can be obtained from a local processing plant. The system should begin at the esophagus and end with the anus. It should be kept frozen until the dissection is performed. Six different colors of Play-Doh Ag Science I – Introduction to Animal Nutrition Monogastric Digestive System • Page 1 of 7 Supplemental Information 1.