Chapter 10 - Gases
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Unit 1 an Introduction to Fluids
UNIT 1 AN INTRODUCTION TO FLUIDS If the rock is not too heavy, you might just _ it up and carry it. When a person moves a rock, the energy to move the rock is suppl ied by the 6. When a rock fall s from a cliff, then it is gravity that is supplying the _ For a fluid to flow, there (must/need not) be a source of energy. 8. When a pump is forcing fluids through a well-bore, the source of energy for flow is the _ 9. The source of energy for a flowing well is re servoir pressure. A flowing well produces because of the of the fluids in the reservoir. 10. Any substance that can flow and that has no definite shape is a fluid. The oil in this tank (has a definite shape/assumes the shape of the tank), Gas (flows/does not flow) and has (a definite/ an indefinite) shape. 12. Anything that flows and has an indefinite shape is a (liquid/ gas/fluid), 15. The oil and gas found in a reservoir are made up of hydrogen atoms and carbon atoms. Hydrocarbons are substances made up only of atoms of ______ and atoms of _ 17. Water is made up of hydrogen atoms and oxygen atoms (H2O)· 18. Different oils and petroleum gases are made up of different combinations of hydrogen and carbon atoms. 21. A substance, water for example, can exist as a gas, a liquid, or a solid. We can change the liquid water to a gas by adding even more 23 Molecules have attractive forces which hold them together. -

Metric System Conversion Factors1 J
AGR39 Metric System Conversion Factors1 J. Bryan Unruh, Barry J. Brecke, and Ramon G. Leon-Gonzalez2 Area Equivalents 1 Hectare (ha) 2 1 Acre (A) = 10,000 square meters (m ) 2 = 100 are (a) = 43,560 square feet (ft ) = 2.471 acres (A) = 4,840 square yards (yd2) = 0.405 hectares (ha) 1 Square Foot (ft) = 160 square rods (rd2) 2 = 4,047 square meters (m2) = 144 square inches (in ) = 929.03 square centimeters (cm2) 2 1 Acre-inch (ac-in) = 0.0929 square meters (m ) 3 = 102.8 cubic meters (m ) 1 Square Mile (mi) = 27,154 gallons, US (gal) 2 = 3,630 cubic feet (ft3) = 27,878,400 square feet (ft ) = 3,097,600 square yards (yd2) 2 1 Are (a) = 640 square acres (A ) = 2,589,988.11 square meters (m2) = 100 square meters (m2) 2 = 119.6 square yards (yd ) 1 Square Rod (rd) = 0.025 acre (A) = 39,204 square inches (in2) = 272.25 square feet (ft2) 1 Cubic Foot (ft) 2 3 = 30.25 square yards (yds ) = 1,728 cubic inches (in ) = 25.3 square meters (m2) = 0.037 cubic yards (yds3) 3 = 0.02832 cubic meters (cm ) 1 Square Yard (yd) = 28,320 cubic centimeters (cm3) = 9 square feet (ft2) 2 1 Cubic Yard (yd) = 0.836 square meters (m ) = 27 cubic feet (ft3) = 0.764 cubic meters (m3) 1. This document is AGR39, one of a series of the Environmental Horticulture Department, UF/IFAS Extension. Original publication date November 1993. Revised December 2014. Reviewed December 2017. Visit the EDIS website at http://edis.ifas.ufl.edu. -
Manual of Style
Manual Of Style 1 MANUAL OF STYLE TABLE OF CONTENTS CHAPTER 1 GENERAL PROVISIONS ...............3 101.0 Scope .............................................. 3 102.0 Codes and Standards..................... 3 103.0 Code Division.................................. 3 104.0 Table of Contents ........................... 4 CHAPTER 2 ADMINISTRATION .........................6 201.0 Administration ................................. 6 202.0 Chapter 2 Definitions ...................... 6 203.0 Referenced Standards Table ......... 6 204.0 Individual Chapter Administrative Text ......................... 6 205.0 Appendices ..................................... 7 206.0 Installation Standards ..................... 7 207.0 Extract Guidelines .......................... 7 208.0 Index ............................................... 9 CHAPTER 3 TECHNICAL STYLE .................... 10 301.0 Technical Style ............................. 10 302.0 Technical Rules ............................ 10 Table 302.3 Possible Unenforceable and Vague Terms ................................ 10 303.0 Health and Safety ........................ 11 304.0 Rules for Mandatory Documents .................................... 11 305.0 Writing Mandatory Requirements ............................... 12 Table 305.0 Typical Mandatory Terms............. 12 CHAPTER 4 EDITORIAL STYLE ..................... 14 401.0 Editorial Style ................................ 14 402.0 Definitions ...................................... 14 403.0 Units of Measure ............................ 15 404.0 Punctuation -

Fundamentals of Hydraulics: Pressure
PUBLISHED BY THE NATIONAL ENVIRONMENTAL SERVICES CENTER FundamentalsBy Zane Satterfield, of P.Hydraulics: E., NESC Engineering Scientist Pressure Summary Hydraulics is the branch of engineering that focuses on the practical problems of collecting, storing, measuring, transporting, controlling, and using water and other liquids. This Tech Brief is the first of two that will discuss some fundamental hydraulic problems and will focus primarily on pressure. The second will discuss flow. Why is understanding hydraulics important? cubic foot (62.4 lb/ft3); so, one cubic foot (1ft x 1ft x 1ft) of water weighs 62.4 lbs. There are 7.48 gallons in The science of hydraulics is as old as civilization itself. a cubic foot, with each gallon weighing approximately For centuries, engineers have succeeded in making water 8.34 pounds, so 7.48 gal x 8.34 lbs/gal = 62.4 pounds. flow from one place to another with as few hitches as possible. When problems do occur, they are usually In the metric system, the term for weight (force due related to the hydraulics involved in pipe flow. to gravity) is called a Newton (N), and the unit weight of water is 9,800 Newtons per cubic meter (9,800 Liquids in motion produce forces and pressure whenever N/m3). More appropriately, this is expressed as 9.8 the velocity, flow direction, or elevation changes. kilonewtons per cubic meter (9.8 kN/m3), where the Knowing pipe pressure and flow at certain points along prefix kilo stands for 1,000. the pipe’s path can help determine pipe size and capac- ity. -
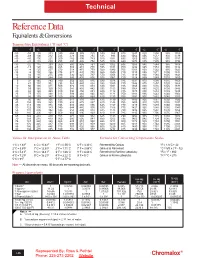
Reference Data Equivalents & Conversions
Technical Reference Data Equivalents & Conversions Temperature Equivalents ( °F and °C) °C °F °C °F °C °F °C °F °C °F °C °F °C °F °C °F -50 -58 95 203 240 464 385 725 530 986 675 1247 820 1508 965 1769 -45 -49 100 212 245 473 390 734 535 995 680 1256 825 1517 970 1778 -40 -40 105 221 250 482 395 743 540 1004 685 1265 830 1526 975 1787 -35 -31 110 230 255 491 400 752 545 1013 690 1274 835 1535 980 1796 -30 -22 115 239 260 500 405 761 550 1022 695 1283 840 1544 985 1805 -25 -13 120 248 265 509 410 770 555 1031 700 1292 845 1553 990 1814 -20 -4 125 257 270 518 415 779 560 1040 705 1301 850 1562 995 1823 -15 -5 130 266 275 527 420 788 565 1049 710 1310 855 1571 1000 1832 -10 14 135 275 280 536 425 797 570 1058 715 1319 860 1580 1005 1841 -5 23 140 284 285 545 430 806 575 1067 720 1328 865 1589 1010 1850 0 32 145 293 290 554 435 815 580 1076 725 1337 870 1598 1015 1859 5 41 150 302 295 563 440 824 585 1085 730 1346 875 1607 1020 1868 10 50 155 311 300 572 445 833 590 1094 735 1355 880 1616 1025 1877 15 59 160 320 305 581 450 842 595 1103 740 1364 885 1625 1030 1886 20 68 165 329 310 590 455 851 600 1112 745 1373 890 1634 1035 1895 25 77 170 338 315 599 460 860 605 1121 750 1382 895 1643 1040 1904 30 86 175 347 320 608 465 869 610 1130 755 1391 900 1652 1045 1913 35 95 180 356 325 617 470 878 615 1139 760 1400 905 1661 1050 1922 40 104 185 365 330 626 475 887 620 1148 765 1409 910 1670 1055 1931 45 113 190 374 335 635 480 896 625 1157 770 1418 915 1679 1060 1940 50 122 195 383 340 644 485 905 630 1166 775 1427 920 1688 1065 1949 55 131 200 -

Pressure Dependence of Senseair´S NDIR Sensors
Pressures Dependence of SenseAir´s NDIR sensors Pressure Dependence of SenseAir´s NDIR sensors Pressure affects the reading of CO 2 sensors. This is because of the physics principle used to measure CO 2 concentration. The NDIR type of sensors measure the IR radiation absorbed by CO 2 molecules, which corresponds to the mole concentration. And when pressure increases, the number of molecules in a given volume also increases linearly. Therefore, all CO 2 sensors have a dependence on pressure. How atmosphere affects the reading of CO 2 Regulation by using ABC function sensors? SenseAir sensors make use of the ABC algorithm to The composition of air is unchanged until an elevation of compensate for any long-term drift due to e.g. aging of approximately 10.000 m, but the atmospheric pressure of components and initial inaccuracy due to e.g. air varies with altitude, that at high altitudes there’s lower transportation. If we located a CO 2 at 2000 m, as figure 2 pressure than at sea level. This means also that the shows, using Senseair’s ABC function can effectively number of molecules per volume unit decreases, adjust the reading close to true reading which minimizes according to the Ideal gas law. the difference generated by pressure sensitivity. Since an NDIR sensor in fact measures the number of molecules per volume unit, it will show a lower reading at higher altitudes. But the unit used by the sensor to express CO 2 concentration is ppm by volume and the volume fraction occupied by CO 2 is the same regardless of pressure, which means that the choice of ppm by volume as unit of measurement for CO 2 sensors introduces a deviation that can be compensated for, if needed. -

EPA Requirements & Refrigerant Handling
Course 5: EPA Requirements & Refrigerant Handling November 4 & 6, 2014 1 Federal Clean Air Act (sc. 608) All persons who maintain, service, repair, or dispose of appliances that contain regulated refrigerants, be cerGfied in proper refrigerant handling techniques. If EPA regulaons change aer a technician becomes cerGfied, it is the responsibility of the technician to comply with any future changes. 2 Technician Certification 4 Categories of Technician Cer1ficaon Type I: Persons who maintain, service or repair small appliances must be cerGfied as Type I technicians. Type II: Persons, who maintain, service, repair or dispose of high or very high-pressure appliances, except small appliances and motor vehicle air condiGoning systems, must be cerGfied as Type II technicians. 3 Technician Certification (cont.) 4 Categories of Technician Cer1ficaon Type III: Persons, who maintain, service, repair, or dispose of low-pressure appliances must be cerGfied as Type III technicians. Type IV (Universal): Persons, who maintain, service or repair both low and high-pressure equipment, as well as small appliances, must be cerGfied as Universal technicians. 4 Refrigeration Definition Heat is a form of energy. Refrigeraon is the movement of heat from an area where it is not wanted to an area where it is less objecGonable. For example, a refrigerator removes heat from the inside of the cabinet and transfers it to the outside. 5 Gauge Manifold Set One of the most important tools to the HVAC&R technician is the gauge manifold set. The compound gauge (BLUE) and the high pressure gauge (RED) are connected to the manifold, and the manifold is then connected by hoses to access ports to measure system pressures. -

Pressure Washing Terminology Acronyms
Pressure Washing Terminology Acronyms: DS - Downstreaming GPM – Gallons per minute PSI – Pound Per Square Inch SH - Sodium hypochlorite Chemicals: Sodium hypochlorite is a chemical compound with the formula NaClO. It is composed of a sodium cation (Na+) and a hypochlorite anion (ClO−); it may also be viewed as the sodium salt of hypochlorous acid. When dissolved in water it is commonly known as bleach or liquid bleach, and is frequently used as a disinfectant or a bleaching agent. Sodium hydroxide, also known as caustic soda, or lye, is an inorganic compound with the chemical formula NaOH. It is a white solid and highly caustic metallic base and alkali salt which is available in pellets, flakes, granules, and as prepared solutions at a number of different concentrations. Sodium hydroxide forms an approximately 50% (by weight) saturated solution with water. Glossary: Downstreaming - Downstream injecting just means that you introduce an additional liquid, such as a detergent, into your power washer through an injector that is mounted after the pump into the high pressure line side. This is usually done in low pressure mode. If you want to do a little high pressure mode injecting in your power washer, you will need an injector designed to operate at high pressures. You'll be happy to know that some of these high pressure injectors can be temporarily installed on the end of your gun lance. Emulsion – A mixture of two or more liquids that are normally immiscible (nonmixable or unblendable). Gallons Per Minute – A measurement of how many gallons of liquid can be supplied through the water pump and a pressure washer. -

Measures Long Measure Avoirdupois Or Commercial Weight 1 Mile = 1760 Yards = 5280 Feet
Evansville, IN 812-477-0077 Henderson, KY 270-830-0077 Jasper, IN 812-482-5105 Owensboro, KY 270-684-0877 Vincennes, IN 812-882-4090 Mt. Vernon, IL 618-244-7156 English Standard Measures Long Measure Avoirdupois or Commercial Weight 1 mile = 1760 yards = 5280 feet. 1 gross or long ton = 2240 pounds. 1 yard = 3 feet = 36 inches. 1 net or short ton = 2000 pounds. 1 foot = 12 inches. 1 pound = 16 ounces = 7000 grains. 1 ounce = 16 drams = 437.5 grains. Surveyor’s Measure 1 mile = 8 furlongs = 80 chains. 1 furlong = 10 chains = 220 yards. Measures of Pressure 1 chain = 4 rods = 22 yards = 66 feet = 100 links. 1 pound per square inch = 144 pounds per square foot = 0.068 1 link = 7.92 inches. atmosphere = 2.042 inches of mercury at 62 degrees F.= 27.7 inches of water at 62 degrees F.= 2.31 feet of water at 62 Square Measure degrees F. 1 square mile = 640 acres = 6400 square chains. 1 atmosphere = 30 inches of mercury at 62 degrees F. = 1 acre = 10 square chains = 4840 square yards = 14.7 pounds per square inch = 2116.3 pounds per square foot = 43,560 square feet. 33.95 feet of water at 62 degrees F. 1 square chain = 16 square rods = 484 square yards = 1 foot of water at 62 degrees F.= 62.355 pounds per square foot = 4356 square feet. 1 square rod = 30.25 square yards = 272.25 square feet = 0.433 pound per square inch. 625 square links. 1 inch of mercury at 62 degrees F.= 1,132 foot of water = 13.58 1 square yard = 9 square feet. -
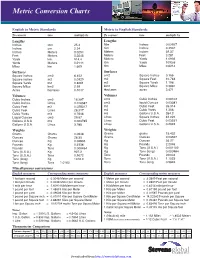
Metric-Conversion-Chart-1
Metric Conversion Charts English to Metric Standards Metric to English Standards To convert into multiply by To convert into multiply by Lengths Lengths Inches mm 25.4 Mm Inches 0.03937 Inches cm 2.54 Cm Inches 0.3937 Inches Meters 0.0254 Meters Inches 39.37 Feet Meters 0.3048 Meters Feet 3.281 Yards km 914.4 Meters Yards 1.0936 Yards Meters 0.9144 Km Yards 1093.6 Miles km 1.609 Km Miles 0.6214 Surfaces Surfaces Square Inches cm2 6.452 cm2 Square Inches 0.155 Square Inches m2 0.0929 m2 Square Feet 10.764 Square Yards m2 0.8361 m2 Square Yards 1.196 Square Miles km2 2.59 km2 Square Miles 0.3861 Acres hectares 0.4047 Hectares acres 2.471 Volumes Volumes Cubic Inches cm3 16.387 cm3 Cubic Inches 0.06102 Cubic Inches Litres 0.016387 cm3 liquid Ounces 0.03381 Cubic Feet m3 0.028317 m3 Cubic Feet 35.314 Cubic Feet Litres 28.317 m3 Cubic Yards 1.308 Cubic Yards m3 0.7646 m3 Gallons U.S.A. 264.2 Liquid Ounces cm3 29.57 Litres Square Inches 61.023 Gallons U.S.A. m3 0.003785 Litres Cubic Feet 0.03531 Gallons U.S.A. Litres 3.785 Litres Gallons U.S.A. 0.2642 Weights Weights Grams Grams 0.0648 Grams grains 15.432 Ounces Grams 28.35 Grams Ounces 0.03527 Ounces Kg 0.02835 Kg Ounces 35.27 Pounds Kg 0.4536 Kg Pounds 2.2046 Pounds Tons 0.000454 Kg Tons (U.S.A.) 0.001102 Tons (U.S.A.) Kg 907.2 Kg Tons (long) 0.000984 Tons (U.S.A.) Tons 0.9072 Tons Pounds 2204.6 Tons (long) Kg 1016.0 Tons Tons (U.S.A.) 1.1023 Tons (long) Tons 1.0160 Tons Tons (long 0.9842 Miscellaneous conversions English measures Corresponding metric measures English measures Corresponding -

Chapter 7. Units and Measurements the SI System (Système International D’Unités) of Reporting Measurements Is Required in All ASA, CSSA, and SSSA Publications
Chapter 7. Units and Measurements The SI system (Système International d’Unités) of reporting measurements is required in all ASA, CSSA, and SSSA publications. Other units may be reported parenthetically if this will clarify interpretation of the data. The National Institute of Standards and Technology maintains online resources for SI (http://physics.nist.gov/cuu/) and has published a comprehensive guide (Thompson & Taylor, 2008) that includes a concise checklist of style requirements. Table 7–6 at the end of this chapter gives selected conversion factors. BASE AND DERIVED UNITS The SI system is based on seven base units (Table 7–1). Derived units (Table 7–2) are expressed algebraically in terms of the base units. Some of these have been given special names and symbols, which may be used to express still other derived units. An example of a derived unit with a special name is the newton (N) for force; the newton is expressed in basic units as m kg s−1. Another unit with a special name is the pascal (Pa), which is one newton per square meter. Using SI Units Publications of ASA, CSSA, and SSSA impose less-stringent requirements in style than the full formal SI system as published by the National Institute of Standards and Technology (Thompson & Taylor, 2008; Taylor & Thompson, 2008), and new develop- ments in SI may take time to win adoption by the editorial boards. For example, this style manual allows molar concentration but disallows normal concentration, whereas strict SI usage declares both to be obsolete (Thompson & Taylor, 2008, 8.6.5). -

Fundamentals of Pressure and Temperature Measurement
Fundamentals of Pressure and Temperature Measurement Jeff Goetzman CenterPoint Energy. 1111 Louisiana Houston, Texas 77002 Our Gauge Pressure (psig) is the gas The correct measuring of Pressure and pressure we read on the meter with an Temperature is one of the most important accurate gauge. For our example we will elements in the accurate measurement of use the common residential delivery Natural Gas. The basic principles were pressure of .25 psi. established many years ago by two men, Robert Boyle an Anglo Irish philosopher, Calculation at .25 psig chemist, and physicist and Jacques Charles, a French Inventor, scientist and Now let’s plug our numbers into our formula: mathematician. Fp = (.25psig+14.7atm) / 14.95base Since we are discussing Fundamentals we will try to keep it as simple possible. For this example the answer would be 1.0. Therefore anytime we measure at these Boyles Law factors we can just simply read the meter index difference as the volume. In 1662 Robert Boyle first published what today is known as Boyles Law. This law can Calculation at 5.0 psig be simply stated as: For a fixed amount of ideal gas kept at a fixed temperature, In this example we will keep everything Pressure [P] and Volume [V] are inversely constant except pressure. Let’s say the proportional. So as pressure is raised more customer called and need more pressure for gas can occupy the same space. a new pool heater or standby generator they installed. As a result a Service Tech was Simple Pressure Factor dispatched and raised the pressure on the meter to 5.0 psig.