The Spontaneous Decomposition Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Potassium Hydroxide Cas N°: 1310-58-3
OECD SIDS POTASSIUM HYDROXIDE FOREWORD INTRODUCTION POTASSIUM HYDROXIDE CAS N°: 1310-58-3 UNEP PUBLICATIONS 1 OECD SIDS POTASSIUM HYDROXIDE SIDS Initial Assessment Report For SIAM 13 Bern, Switzerland, 6-9 November 2001 1. Chemical Name: Potassium hydroxide 2. CAS Number: 1310-58-3 3. Sponsor Country: Belgium Dr. Thaly LAKHANISKY J. Wytsman 16 B-1050 Brussels, Belgium Tel : + 32 2 642 5104 Fax : +32 2 642 5224 E-mail : [email protected] 4. Shared Partnership with: ICCA (Tessenderlo Chemie NV) 5. Roles/Responsibilities of the Partners: · Name of industry sponsor /consortium · Process used 6. Sponsorship History · How was the chemical or In 2001, ICCA (Tessenderlo Chemie NV)) had proposed sponsor category brought into the and prepared draft documents(Dossier, SIAR, SIAP). It was OECD HPV Chemicals submitted to the SIDS contact point of Belgium on May 2001. Programme? The draft documents were revised by Belgium after discussion with Tessenderlo Chemie NV. The revised draft was discussed in detail with Tessenderlo Chemie NV on June and July 2001. After agreement, the documents were finalized and the checklist was developed by jointly by Belgium and Tessenderlo Chemie NV 7. Review Process Prior to the SIAM: 8. Quality check process: 9. Date of Submission: 10. Date of last Update: February 2002 11. Comments: No testing 2 UNEP PUBLICATIONS OECD SIDS POTASSIUM HYDROXIDE SIDS INITIAL ASSESSMENT PROFILE CAS No. 1310-58-3 Chemical Name Potassium hydroxide Structural Formula KOH RECOMMENDATIONS The chemical is currently of low priority for further work. SUMMARY CONCLUSIONS OF THE SIAR Human Health Solid KOH is corrosive. -

Sodium Chlorate Process Liquor De-Chromed SN
SAFETY DATA SHEET This SDS adheres to the standards and regulatory requirements of the United States and may not meet the regulatory requirements in other countries. 1. Identification Product identifier Sodium Chlorate Process Liquor De-chromed SN Other means of identification De-chromed blend of Crystallizer Feed Liquor and Mother Liquor, NaClO3 Recommended use For internal transfer between ERCO Worldwide sodium chlorate manufacturing facilities for process purposes Recommended restrictions None known Manufacturer/Importer/Supplier/Distributor information Manufacturer Company name ERCO Worldwide Address 335 Carlingview Drive Unit 1 Etobicoke, M9W 5G8 Canada Telephone Information #: (416) 239-7111 (M- F: 8:00 am – 5:00pm EST) Website http://www.ercoworldwide.com E-mail [email protected] Emergency phone number Canada & USA: 1-800-424-9300 (CHEMTREC) Supplier Refer to Manufacturer 2. Hazard(s) Identification Physical hazards Oxidizing liquids Category 2 Health hazards Acute toxicity, oral Category 4 Environmental hazards Not currently regulated by OSHA, refer to Section 12 for additional information. OSHA defined hazards This mixture does not meet the classification criteria according to OSHA HazCom 2012. Label elements Signal word Danger Hazard statement May intensify fire; oxidizer. Harmful if swallowed. Page 1 of 15 Issue Date: 11/18/2020 Sodium Chlorate Process Liquor De-chromed SN Precautionary statement Prevention Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. Keep away from clothing and other combustible materials. Wear protective gloves, protective clothing, eye protection, face protection. Response IF ON SKIN: Wash with plenty of water. Take off contaminated clothing and wash it before reuse. In case of fire: Use water to extinguish. -

Common Name: POTASSIUM CHLORATE HAZARD SUMMARY IDENTIFICATION REASON for CITATION HOW to DETERMINE IF YOU ARE BEING EXPOSED WORK
Common Name: POTASSIUM CHLORATE CAS Number: 3811-04-9 DOT Number: UN 1485 RTK Substance number: 1560 UN 2427 (Aqueous solution) Date: March 1998 Revision: October 2004 --------------------------------------------------------------------------- --------------------------------------------------------------------------- HAZARD SUMMARY WORKPLACE EXPOSURE LIMITS * Potassium Chlorate can affect you when breathed in. No occupational exposure limits have been established for * Contact can cause eye and skin irritation and burns. Potassium Chlorate. This does not mean that this substance * Breathing Potassium Chlorate can irritate the nose, throat is not harmful. Safe work practices should always be and lungs causing sneezing, coughing and sore throat. followed. * High levels can interfere with the ability of the blood to carry oxygen causing headache, weakness, dizziness and a WAYS OF REDUCING EXPOSURE blue color to the skin (methemoglobinemia). Higher levels * Where possible, enclose operations and use local exhaust can cause trouble breathing, collapse and even death. ventilation at the site of chemical release. If local exhaust * Repeated exposure may affect the kidneys and nervous ventilation or enclosure is not used, respirators should be system. worn. * Wear protective work clothing. IDENTIFICATION * Wash thoroughly immediately after exposure to Potassium Chlorate is a transparent, colorless crystal or Potassium Chlorate and at the end of the workshift. white powder. It is used as an oxidizing agent, and in * Post hazard and warning information in the work area. In explosives, matches, textile printing, disinfectants and addition, as part of an ongoing education and training bleaches. effort, communicate all information on the health and safety hazards of Potassium Chlorate to potentially REASON FOR CITATION exposed workers. * Potassium Chlorate is on the Hazardous Substance List because it is cited by DOT. -

Production of Hexavalent Chromium for Use in Chlorate Cells
Europaisches Patentamt J European Patent Office © Publication number: 0 266 128 A2 Office europeen des brevets EUROPEAN PATENT APPLICATION © Application number: 87309335.5 © Int. CIA C25B 1/26 @ Date of filing: 22.10.87 ® Priority: 29.10.86 CA 521737 © Applicant: Tenneco Canada Inc. 2 Gibbs Road © Date of publication of application: Islington OntarioM9B 1R1(CA) 04.05.88 Bulletin 88/18 0 Inventor: Dobosz, Leszek Michal © Designated Contracting States: 68 MacDonald Street CH ES FR LI SE Toronto Ontario M8V 1Y4(CA) © Representative: Hamilton, Raymond et al c/o Albright & Wilson Limited 1 Knightsbridge Green London SW1X 7QD(GB) © Production of hexavalent chromium for use in chlorate cells. © By-product hypochlorite from the electrolytic production of chlorates, notably sodium chlorate, is used to form hexavalent chromium for use in the electrolysis process by oxidation of trivalent chromium compounds by the hypochlorite. The hypochlorite maybe the condensate produced by treatment of the chlorate cell by-product gas stream and/or present in the cell liquor. < 00 CO CO (VI a. UJ Xerox Copy Centre 0 266 128 PRODUCTION OF HEXAVALENT CHROMIUM FOR USE IN CHLORATE CELLLS The present invention relates to the fomation of hexavalent chromium useful in the electrolytic production of aqueous chlorate solutions. An aqueous solution of sodium chlorate and sodium chloride is conventionally produced by the electrolysis of aqueous sodium chloride in diaphragmless electrolytic cells. The extent of electrolysis is 5 controlled to produce an effluent from the cell in which the sodium chlorate and sodium chloride have the desired ratio, usually in the range (expressed as a weight ratio) of about 1 :1 to about 20:1 and preferably in the range of about 2:1 to about 15:1. -

Guidelines for Drinking-Water Quality, Fourth Edition
12. CHEMICAL FACT SHEETS Assessment date 1993 Principal reference WHO (2003) Chlorine in drinking-water In humans and experimental animals exposed to chlorine in drinking-water, no specific adverse treatment-related effects have been observed. IARC has classified h ypochlorite in Group 3 (not classifiable as to its carcinogenicity to humans). Chlorite and chlorate Chlorite and chlorate are disinfection by-products resulting from the use of chlorine dioxide as a disinfectant and for odour and taste control in water. Chlorine dioxide is also used as a bleaching agent for cellulose, paper pulp, flour and oils. Sodium chlorite and sodium chlorate are both used in the production of chlorine dioxide as well as for other commercial purposes. Chlorine dioxide rapidly decomposes into chlorite, chlorate and chloride ions in treated water, chlorite being the predominant species; this reaction is favoured by alkaline conditions. The major route of environmental ex- posure to chlorine dioxide, sodium chlorite and sodium chlorate is through drinking- water. Chlorate is also formed in sodium hypochlorite solution that is stored for long periods, particularly at high ambient temperatures. Provisional guideline values Chlorite: 0.7 mg/l (700 µg/l) Chlorate: 0.7 mg/l (700 µg/l) The guideline values for chlorite and chlorate are designated as provisional because use of chlorine dioxide as a disinfectant may result in the chlorite and chlorate guideline values being exceeded, and difficulties in meeting the guideline value must never be a reason for compromising adequate disinfection. Occurrence Levels of chlorite in water reported in one study ranged from 3.2 to 7.0 mg/l; however, the combined levels will not exceed the dose of chlorine dioxide applied. -

Toasting a Gummy Candy
WARNING NOTICE The experiments described in these materials are potentially hazardous. Among other things, the experiments should include the following safety measures: a high level of safety training, special facilities and equipment, the use of proper personal protective equipment, and supervision by appropriate individuals. You bear the sole responsibility, liability, and risk for the implementation of such safety procedures and measures. MIT and Dow shall have no responsibility, liability, or risk for the content or implementation of any of the material presented. Legal Notice TOASTING A GUMMY CANDY A photo taken at MIT 150th Celebration Open House Under the Dome April 30, 2011. Courtesy of Nathan Sanders. Used with permission. Abstract A gummy bear candy is oxidized using KClO3 as the oxidizing agent in a dramatic reaction, which releases a large amount of energy and results in the formation of harmless products KCl, CO2, and H2O. Materials Gummy Bear Candy 25mm Heavy-Walled Borosilicate Test Tube Potassium chlorate, KClO3 Cast Iron Support Stand for Test Tube Clamp Spatula Test Tube Clamp Explosion Shield Portable Butane Burner or Lab Burner Long laboratory tweezers Face shield Safety Potassium chlorate is a strong oxidizer, which could ignite if placed into contact with other materials. It is a known skin, eye, and respiratory tract irritant. Potassium chlorate should always be fresh and weighed on a balance right before using it. It’s a good idea to mass it directly into the test tube you are going to use in this experiment and cover the opening with paraffin wax paper to protect the contents from any contamination. -

Each Lesson Section Is Divided Into Four Segmentsobjectives, a List of References, Suggested Activities, and ? Content Outline of the Material
DOCUMENT P7SUME ED 049 151 SP 007 022 AUTHOR Eeyer, Frederick Spooner, William Y. TITLE Earth Science. In-Seivice Television Program. INSTITUTION North Carolina State Board of Education, Raleigh. Dept.of Public Instruction. PUB DATE [70] NOTE 325p. EDRS PRICE EDRS Price MF-$0.65 HC-$13.16 DESCRIPTORS *Curriculum Guides, *Earth Science, Geoloyy, *Inservice Teacher Education, Meteorology, Oceanology, *Physical Geography, ',Se,,:ondary Education, Soil Science ABSTRACT GRADES OR AGES: Inservice course for secondary teacners. SUBJECT MATTER: Earth science. ORGANIZATION AND PHYSICAL APPEARANCE: The guide is intended for use with a 32- program television course f)c teachers, with material intended to be used in tne classroom. The introductory material explains the rationale of the course and includes the transmission schedule and bibliography. Each lesson section is divided into four segmentsobjectives, a list of references, suggested activities, and ? content outline of the material. The guide is offset printed and is in a looseleaf binder- OBJECTIVE:. AND ACTIVITIES: The o'cjectives are listed at the beginning of each lesson. Suggested activities arG included in each lesson and are intended to Provide examples of student investigation, demonstrations, or activities which will demonstrate 3 particular concept. INSTRUCTIONAL MATSFIALS: References to relevant printed material are given In each lesson, and other materials ate also referred to in the text. STUDENT ASSESSMENT: No provision is trade for evaluation. (MBM) US OF.TARTMENI OF HEALTH. EN/CA.710N & WE:JAME OFFICE OF EDUCATION THIS DOCLNIENT HAS BEEN REPRO- DUCED EXACTLY AS RECEIVED FROM THE PERSON OR ORGANIZATION OHIG :HATING IT POINTS OF VIEW OR 0.IN ,ONS STATED GO NOT NECESSARILY REPRESENT OFFICIAL OFF/CE OF EDU- CATION POSITION OR POLICY EARTH SCIENCE IN- SERVICE TELEVISION PROGRAM NORTH CAROLINA DEPARTMENT OF PUBLIC INSTRUCTION /RALEIGH PREPARED AND PRESENTED BY FREDERICK L. -
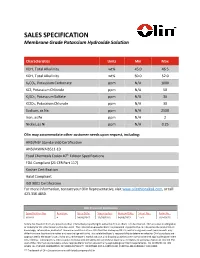
SALES SPECIFICATION Membrane Grade Potassium Hydroxide Solution
SALES SPECIFICATION Membrane Grade Potassium Hydroxide Solution Characteristics Units Min Max KOH, Total Alkalinity wt% 45.0 46.5 KOH, Total Alkalinity wt% 50.0 52.0 K2CO3, Potassium Carbonate ppm N/A 1000 KCl, Potassium Chloride ppm N/A 50 K2SO4, Potassium Sulfate ppm N/A 30 KClO3, Potassium Chlorate ppm N/A 30 Sodium, as Na ppm N/A 2500 Iron, as Fe ppm N/A 2 Nickel, as Ni ppm N/A 0.25 Olin may accommodate other customer needs upon request, including: ANSI/NSF Standard 60 Certification ANSI/AWWA B511-10 Food Chemicals Codex 10th Edition Specifications FDA Compliant (21 CFR Part 117) Kosher Certification Halal Compliant ISO 9001 Certification For more information, contact your Olin Representative, visit www.olinchloralkali.com, or call 423.336.4850. Olin Document Information Specification No: Revision: Issue Date: Supersedes: Review Date: Sheet No.: Form No.: KOH-S1 4 04/18/2017 12/28/2016 04/18/2022 1 of 1 102-00526 Notice: No freedom from any patent or other intellectual property rights owned by Olin or others is to be inferred. Olin assumes no obligation or liability for the information in this document. The information provided herein is presented in good faith a nd is based on the best of Olin’s knowledge, information, and belief. Since use conditions at non-Olin facilities are beyond Olin’s control and government requirements may differ from one location to another and may change with time, it is solely the Buyer’s responsibility to determine whether Olin’s products are appropriate for the Buyer’s use, and to assure the Buyer’s workplace, use, and disposal practices are in compliance with appl icable government requirements. -

Dangers of Unspent Aircraft Oxygen Generators
Safety Advisory Dangers of Unspent Aircraft Oxygen Generators U.S. Chemical Safety and Hazard Investigation Board No. 2007-I-NC-01-SA | June 2007 Key Message This Safety Advisory is issued to alert aircraft maintenance and hazardous waste facility personnel to the hazards associated with the transportation and storage of expired, unspent aircraft chemical oxygen generators. Aircraft oxygen generators are dangerous devices that, if mishandled, can cause fires, property damage, and personal injury. Aircraft oxygen generators that have exceeded their service life should be expended before shipping by any transport mode. Introduction On October 5, 2006, at about 10 pm, a fire occurred at the EQ Industrial Services (EQ) hazardous waste treatment, storage, and disposal facility in Apex, North Carolina. The fire resulted in the evacuation of thousands of Apex residents and the complete destruction of the hazardous waste building at EQ’s Apex facility. The U.S. Chemical Safety and Hazard Investigation Board (CSB) investigation concluded that aircraft oxygen generators most likely contributed to the rapid spread of the fire to other areas in the EQ facility. The CSB issues this Safety Advisory to focus attention on the need for aircraft maintenance facilities to expend chemical oxygen generators that have exceeded their service life, and for hazardous waste facility operators and shippers to exercise due care when handling unspent chemical oxygen generators. Incident Description At about 10 pm on October 5, 2006, a citizen driving past the EQ facility in Apex, North Carolina, called 911 when he saw a plume of smoke and smelled a strong chlorine odor coming from the facility. -

Prohibited and Restricted Chemical List
School Emergency Response Plan and Management Guide Prohibited and Restricted Chemical List PROHIBITED AND RESTRICTED CHEMICAL LIST Introduction After incidents of laboratory chemical contamination at several schools, DCPS, The American Association for the Advancement of Science (AAAS) and DC Fire and Emergency Management Services developed an aggressive program for chemical control to eliminate student and staff exposure to potential hazardous chemicals. Based upon this program, all principals are required to conduct a complete yearly inventory of all chemicals located at each school building to identify for the removal and disposal of any prohibited/banned chemicals. Prohibited chemicals are those that pose an inherent, immediate, and potentially life- threatening risk, injury, or impairment due to toxicity or other chemical properties to students, staff, or other occupants of the school. These chemicals are prohibited from use and/or storage at the school, and the school is prohibited from purchasing or accepting donations of such chemicals. Restricted chemicals are chemicals that are restricted by use and/or quantities. If restricted chemicals are present at the school, each storage location must be addressed in the school's written emergency plan. Also, plan maps must clearly denote the storage locations of these chemicals. Restricted chemicals—demonstration use only are a subclass in the Restricted chemicals list that are limited to instructor demonstration. Students may not participate in handling or preparation of restricted chemicals as part of a demonstration. If Restricted chemicals—demonstration use only are present at the school, each storage location must be addressed in the school's written emergency plan. Section 7: Appendices – October 2009 37 School Emergency Response Plan and Management Guide Prohibited and Restricted Chemical List Following is a table of chemicals that are Prohibited—banned, Restricted—academic curriculum use, and Restricted—demonstration use only. -

Six-Year Review 3 Technical Support Document for Chlorate
Six-Year Review 3 Technical Support Document for Chlorate Office of Water (4607M) EPA-810-R-16-013 December 2016 Disclaimer This document is not a regulation. It is not legally enforceable, and does not confer legal rights or impose legal obligations on any party, including EPA, states, or the regulated community. While EPA has made every effort to ensure the accuracy of any references to statutory or regulatory requirements, the obligations of the interested stakeholders are determined by statutes, regulations or other legally binding requirements, not this document. In the event of a conflict between the information in this document and any statute or regulation, this document would not be controlling. This page intentionally left blank. Table of Contents 1 Introduction ................................................................................................................. 1-1 2 Contaminant Background .......................................................................................... 2-1 2.1 Chemical and Physical Properties ................................................................................. 2-1 2.2 Production, Use and Release ......................................................................................... 2-2 2.2.1 Commercial Production and Use in Industry and Agriculture ........................... 2-2 2.2.2 Incidental Production and Release ...................................................................... 2-6 2.3 Environmental Fate ...................................................................................................... -
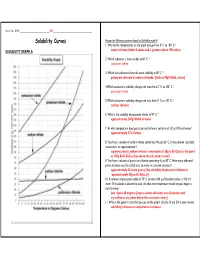
Solubility Curves Answer the Following Questions Based on Solubility Graph a 1
Name, Date, Hr/Per_______________________________ KEY ________________________________________ Solubility Curves Answer the following questions based on Solubility graph A 1. Why do the temperatures on the graph only go from 0º C to 100º C ? SOLUBILITY GRAPH A water is frozen below 0 celcus and is gaseous above 100 celcius 2. Which substance is most soluble at 60º C ? potassium iodide 3. Which two substances have the same solubility at 80º C ? potassium chlorate & sodium chloride [both at 40g/100mL water] 4.Which substance’s solubility changes the most from 0º C to 100º C ? potassium nitrate 5.Which substance’s solubility changes the least from 0º C to 100º C ? sodium chloride 6. What is the solubility of potassium nitrate at 90º C ? approximately 200g/100mL of water 7. At what temperature does potassium iodide have a solubility of 150 g/ 100 cm3 water? approximately 22.5 Celcius 8. You have a solution of sodium nitrate containing 140 g at 65º C. Is the solution saturated, unsaturated, or supersaturated ? supersaturated [sodium nitrate is saturated at 130g at 65 Celcius; the point at 140g & 65 Celcius lies above the saturation curve] 9. You have a solution of potassium chlorate containing 4 g at 65º C. How many additional grams of solute must be added to it, to make the solution saturated ? approximately 26 more grams [the solubility of potassium chlorate is `approximately 30g at 65 Celcius] 10. A solution of potassium iodide at 70º C contains 200 g of dissolved solute in 100 cm 3 water. The solution is allowed to cool.