The Insulator-Metal Transition in Hydrogen
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recent Studies in Superconductivity at Extreme Pressures
Recent Studies in Superconductivity at Extreme Pressures James S. Schilling and James J. Hamlin Department of Physics, Washington University CB 1105, One Brookings Dr, St. Louis, MO 63130, USA October 12, 2007 Abstract Studies of the effect of high pressure on superconductivity began in 1925 with the seminal work of Sizoo and Onnes on Sn to 0.03 GPa and have continued up to the present day to pressures in the 200 - 300 GPa range. Such enormous pressures cause profound changes in all condensed matter properties, including superconductivity. In high pressure experiments metallic elements, Tc values have been elevated to temperatures as high as 20 K for Y at 115 GPa and 25 K for Ca at 160 GPa. These pressures are sufficient to turn many insulators into metals and magnetics into superconductors. The changes will be particularly dramatic when the pressure is sufficient to break up one or more atomic shells. Recent results in superconductivity to Mbar pressures wll be discussed which exemplify the progress made in this field over the past 82 years. 1 Superconductivity is a macroscopic quantum phenomenon which was discovered by G. J. Holst and H. K. Onnes [1] in Leiden in 1911, but not clearly understood untilBardeen,Cooper,andSchrieffer (BCS) [2] formulated their microscopic theory in 1957, exactly half a century ago. In the opinion of one of the present authors (JSS), had superconductivity not first been demonstrated in experiment, no theorist would have ever predicted it: who could imagine that two electrons, in spite of their Coulomb repulsion, might experience a net attractive interaction binding them together to form a bose particle? In a lecture in 1922 in honor of H. -

Ternary Superconducting Cophosphorus Hydrides Stabilized Via Lithium
www.nature.com/npjcompumats ARTICLE OPEN Ternary superconducting cophosphorus hydrides stabilized via lithium Ziji Shao1, Defang Duan 1*, Yanbin Ma2, Hongyu Yu1, Hao Song1, Hui Xie1,DaLi 1, Fubo Tian1, Bingbing Liu1 and Tian Cui1* Inspired by the diverse properties of sulfur hydrides and phosphorus hydrides, we combine first-principles calculations with structure prediction to search for stable structures of Li−P−H ternary compounds at high pressures with the aim of finding novel superconductors. It is found that phosphorus hydrides can be stabilized under pressure via additional doped lithium. Four stable stoichiometries LiPH3, LiPH4, LiPH6, and LiPH7 are uncovered in the pressure range of 100–300 GPa. Notably, we find an atomic LiPH6 with Pm3 symmetry which is predicted to be a potential high-temperature superconductor with a Tc value of 150–167 K at 200 GPa and the Tc decreases upon compression. All the predicted stable ternary hydrides contain the P–H covalent frameworks with ionic lithium staying beside, but not for Pm3-LiPH6. We proposed a possible synthesis route for ternary lithium phosphorus hydrides: LiP + H2 → LiPHn, which could provide helpful and clear guidance to further experimental studies. Our work may provide some advice on further investigations on ternary superconductive hydrides at high pressure. npj Computational Materials (2019) 5:104 ; https://doi.org/10.1038/s41524-019-0244-6 1234567890():,; INTRODUCTION Experimentally, the PH3 is found to be gradually decomposed Given the metallization and potential room temperature super- into P2H4,P4H6, and then further into elemental phases above 22,23 conductivity of solid hydrogen under high pressure have been 35 GPa at room temperature. -

Download This Article PDF Format
RSC Advances PAPER View Article Online View Journal | View Issue Pressure-induced metallization in MoSe2 under different pressure conditions† Cite this: RSC Adv.,2019,9,5794 Linfei Yang,ab Lidong Dai, *a Heping Li,a Haiying Hu,a Kaixiang Liu,ab Chang Pu,ab Meiling Hongab and Pengfei Liuc In this study, the vibrational and electrical transport properties of molybdenum diselenide were investigated under both non-hydrostatic and hydrostatic conditions up to 40.2 GPa using the diamond anvil cell in conjunction with Raman spectroscopy, electrical conductivity, high-resolution transmission electron microscopy, atomic force microscopy, and first-principles theoretical calculations. The results obtained indicated that the semiconductor-to-metal electronic phase transition of MoSe2 can be extrapolated by some characteristic parameters including abrupt changes in the full width at half maximum of Raman modes, electrical conductivity and calculated bandgap. Under the non-hydrostatic condition, metallization occurred at 26.1 GPa and it was irreversible. However, reversible metallization occurred at 29.4 GPa under the hydrostatic condition. In addition, the pressure-induced metallization reversibility Creative Commons Attribution 3.0 Unported Licence. Received 16th November 2018 of MoSe can be revealed by high-resolution transmission electron and atomic force microscopy of the Accepted 4th February 2019 2 recovered samples under different hydrostatic conditions. This discrepancy in the metallization DOI: 10.1039/c8ra09441a phenomenon of MoSe2 in different hydrostatic environments was attributed to the mitigated interlayer rsc.li/rsc-advances van der Waals coupling and shear stress caused by the insertion of pressure medium into the layers. Introduction understanding the crystalline structure evolution and electrical properties of AB2-type layered materials, and also promotes its ¼ ¼ The AB2-type (A Mo, W; B S, Se, Te) transition-metal industrial exploitation in electronic devices. -

Liquid Metallic Hydrogen at Static Conditions
Liquid Metallic Hydrogen at Static Conditions The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:40046435 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA A DISSERTATION PRESENTED BY MOHAMED ZAGHOO To THE JOHN PAULSON SCHOOL OF ENGINEERING AND APPLIED SCIENCE IN PARTIAL FULLFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSIPHY IN THE SUBJECT OF APPLIED PHYSICS HARVARD UNIVERSITY CAMBRIDGE, MASSACHUSETTS January 2017 ©2017 – MOHAMED ZAGHOO All RIGHTS RESERVED THESIS ADVISOR: PROFESSOR ISAAC F. SILVERA AUTHOR: MOHAMED ZAGHOO LIQUID METALLIC HYDROGEN AT STATIC CONDITIONS Abstract The search for dense hydrogen metallization is now almost eighty years old. Because of hydrogen’s fundamental significance as the benchmark system for most of the physical and chemical sciences as well as its astrophysical abundance, the problem has been dubbed as the holy grail of high-pressure physics. Despite a legion of experimental feats and a remarkable theoretical progress over the last century, the precise conditions, mechanism and nature of this metallization process remain elusive. This thesis reports the first production of metallic hydrogen in the liquid phase at bench-top experiments under static conditions and high temperatures. The nature of metallization and the mechanism of conduction are shown to be different than hitherto assumed. -
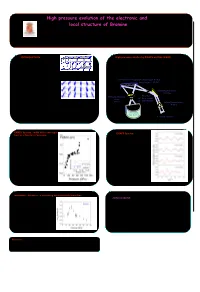
High Pressure Evolution of the Electronic and Local Structure of Bromine
High pressure evolution of the electronic and local structure of Bromine A.San Miguel1, H. Libotte2 , G. Aquilanti3, S. Pascarelli3, and J.-P. Gaspard2 1 Physique de la Matière Condensée et Nanostructures,University of Lyon 1, France 2 Condensed Matter Physics Lab., University of Liège, Belgium 3 ESRF, Grenoble, France INTRODUCTION The structure of iodine High pressure studies by EXAFS on ID24 (ESRF) The halogens forms diatomic molecules and molecular crystals: the intra-molecular distance The XAS is the ideal technique to study the structural and electronic is much smaller than any other interatomic properties of systems under high pressures as it the most sensitive tool to distances within the crystal. Among the measure the short interatomic distances (EXAFS) and a valuable probe to molecular crystals, bromine is one of the measure the width of the unoccupied conduction band (XANES). We have simplest. In spite of this simplicity it still studied the high pressure evolution of the bromine molecular crystal using the Top : simple cubic structure (P < 80 Gpa) reserves many hidden surprises[1]. high pressure dedicated ID24 beamline at ESRF, sketched below. Bottom : structure of Br2 (schematic) The formation of diatomic molecules is the Si(111) or Si(311) Bragg/Laue polychromator @ 64 m result of a Peierls distortion of a simple cubic Energy range: 5 - 28 KeV structure. One distance is shortened and the remaining five distances are elongated is as shown Demagnification mirror schematically. This is an example of the Octet @ 32.5 m rule. Vertically refocusing Focal Spot mirror Δx ~ 15 µm FWHM a) The pressure is known to destroy the Peierls Δz ~ 15 µm FWHM @ 65 m Vertical focusing mirror distortion like e. -

Dynamic High Pressure: Why It Makes Metallic Fluid Hydrogen
Dynamic high pressure: Why it makes metallic fluid hydrogen W. J. Nellis Department of Physics Harvard University Metallic fluid H has been made by dynamic compression decades after Wigner and Huntington (WH) predicted its existence in 1935. The density obtained experimentally is within a few percent of the density predicted by WH. Metallic fluid H was achieved by multiple-shock compression of liquid H2, which compression is quasi-isentropic and H is thermally equilibrated in experiments with 100 ns lifetimes. Quasi-isentropic means compressions are isentropic but with sufficient temperature and entropy to drive a crossover from liquid H2 to degenerate fluid H at 9-fold compression and pressure P=140 GPa (1.4 Mbar). The metallic fluid is highly degenerate: T/TF ≈ 0.014, where temperature T=3000 K and Fermi temperature TF =220,000 K, respectively, at metallization density 0.64 mol H/cm3. Dynamic compression is achieved by supersonic, adiabatic, nonlinear hydrodynamics, the basic ideas of which were developed in the Nineteenth Century in European universities. Today dynamic compression is generally unfamiliar to the scientific community. The purposes of this paper are to present a brief review of (i) dynamic compression and its affects on materials, (ii) considerations that led to the sample holder designed specifically to make metallic fluid H, and (iii) an inter-comparison of dynamic and static compression with respect to making metallic H. 1 I. Introduction In 1935 Wigner and Huntington (WH) predicted that solid insulating H2 would undergo a 3 transition to metallic H at a density 0.62 mol H/cm (9-fold liquid-H2 density), “very low temperatures”, and a pressure greater than 25 GPa [1]. -

Pressure-Induced Bonding and Compound Formation in Xenon–Hydrogen Solids Maddury Somayazulu1*, Przemyslaw Dera2, Alexander F
Click here for supplementary data ARTICLES PUBLISHED ONLINE: 22 NOVEMBER 2009 | DOI: 10.1038/NCHEM.445 Pressure-induced bonding and compound formation in xenon–hydrogen solids Maddury Somayazulu1*, Przemyslaw Dera2, Alexander F. Goncharov1, Stephen A. Gramsch1, Peter Liermann3,WengeYang3,ZhenxianLiu1,Ho-kwangMao1,3 and Russell J. Hemley1 Closed electron shell systems, such as hydrogen, nitrogen or group 18 elements, can form weakly bound stoichiometric compounds at high pressures. An understanding of the stability of these van der Waals compounds is lacking, as is information on the nature of their interatomic interactions. We describe the formation of a stable compound in the Xe–H2 binary system, revealed by a suite of X-ray diffraction and optical spectroscopy measurements. At 4.8 GPa, a unique hydrogen-rich structure forms that can be viewed as a tripled solid hydrogen lattice modulated by layers of xenon, consisting of xenon dimers. Varying the applied pressure tunes the Xe–Xe distances in the solid over a broad range from that of an expanded xenon lattice to the distances observed in metallic xenon at megabar pressures. Infrared and Raman spectra indicate a weakening of the intramolecular covalent bond as well as persistence of semiconducting behaviour in the compound to at least 255 GPa. ydrogen occupies a unique position in the periodic table as a group R3. The structure of the xenon sublattice could be successfully result of its quantum nature and simple electronic structure, solved using direct methods, which resulted in an excellent refine- ¼ Hand the prediction of unusual chemical, electronic and dyna- ment (Rl 3.78%) for 120 unique observed reflections (see mical properties at very high pressures. -

Opacity and Conductivity Measurements in Noble Gases at Conditions of Planetary and Stellar Interiors R
Opacity and conductivity measurements in noble gases at conditions of planetary and stellar interiors R. Stewart McWilliamsa,b,c,d,e,1, D. Allen Daltona, Zuzana Konôpkováf, Mohammad F. Mahmooda,e, and Alexander F. Goncharova,g aGeophysical Laboratory, Carnegie Institution of Washington, Washington, DC 20015; bSchool of Physics and Astronomy, University of Edinburgh, Edinburgh EH9 3FD, United Kingdom; cCentre for Science at Extreme Conditions, University of Edinburgh, Edinburgh EH9 3FD, United Kingdom; dDepartamento de Geociencias, Universidad de Los Andes, Bogotá DC, Colombia; eDepartment of Mathematics, Howard University, Washington, DC 20059; fDeutsches Elektronen-Synchrotron Photon Science, 22607 Hamburg, Germany; and gKey Laboratory of Materials Physics, Institute of Solid State Physics, Chinese Academy of Sciences, Hefei 230031, China Edited by Vladimir E. Fortov, Russian Academy of Sciences, Moscow, Russia, and approved May 15, 2015 (received for review November 13, 2014) The noble gases are elements of broad importance across science Here we report experiments in the laser-heated diamond anvil and technology and are primary constituents of planetary and cell (15, 16, 26–29) on high-density and high-temperature states stellar atmospheres, where they segregate into droplets or layers of the noble gases Xe, Ar, Ne, and He (Fig. 1). Rapid heating that affect the thermal, chemical, and structural evolution of their and cooling of compressed samples using pulsed laser heating host body. We have measured the optical properties of noble (26, 27) is coupled with time domain spectroscopy of thermal gases at relevant high pressures and temperatures in the laser- emission (26) to determine sample temperature and transient heated diamond anvil cell, observing insulator-to-conductor trans- absorption to establish corresponding sample optical properties formations in dense helium, neon, argon, and xenon at 4,000– (Figs. -

Chapter 4: a Case Study (Hydrogen)
4. A Case Study: Hydrogen 39 4 A Case Study: Hydrogen The most abundant planetary ingredient is hydrogen. In this chapter we discuss the behavior of this material. However, we will also use this case study as an opportunity to introduce a number of basic ideas involving phase transitions. In reality, even this simplest of elements turns out to be remarkably complicated and the approach described here is inadequate. However, it still serves as a valuable introduction to the general ideas. 4.1 What is the Zero Pressure Ground State of Hydrogen? In the last chapter, we calculated the energy for a uniform ensemble of hydrogen as protons and electrons. The zero-pressure energy for this state (when computed as carefully as possible; i.e., including correlation and band structure) is about -1.03 or -1.04 Rydbergs per hydrogen (or equivalently, per electron), so it is bound relative to hydrogen atoms (which lie at exactly –1.00 Rydberg). It is not, however, lower than the energy per proton of molecular hydrogen gas, liquid, or solid, where the energy is about -1.15 Ryd. (It takes about 4 eV, or 0.3 Ryd, to bust up a hydrogen molecule.) So hydrogen prefers to form molecules at zero pressure. (This is not true of other first column elements in the periodic table, i.e., Li, Na, K...) 4.2 The Molecular-Metallic Transition in Hydrogen; A Traditional View We have seen that hydrogen could exist in a metallic state (free-electron-like state) analogous to alkali metals. Recall that hydrogen is in column one of the periodic table. -

Superconductivity in Boron a Quasi–Four-Electrode Scheme in Which Four 2-M-Thick Pt Electrodes Were Electrically Con- Mikhail I
R EPORTS thickness was placed at the culet. Because of the small size of the sample in this run, we used Superconductivity in Boron a quasi–four-electrode scheme in which four 2-m-thick Pt electrodes were electrically con- Mikhail I. Eremets,1,2 Viktor V. Struzhkin,1 Ho-kwang Mao,1 1 nected in pairs by crossing their tips near the Russell J. Hemley * sample. The sample was then connected to these joined leads with 2-m-thick Pd elec- Metals formed from light elements are predicted to exhibit intriguing states of trodes. Thus, the resistance of the sample was electronic order. Of these materials, those containing boron are of considerable measured in series with the two Pd electrodes. current interest because of their relatively high superconducting temperatures. The sample at ambient pressure showed no We have investigated elemental boron to very high pressure using diamond measurable resistance (R Ͼ 300 Mohm), but anvil cell electrical conductivity techniques. We find that boron transforms from exhibited strong photoconductivity under illu- a nonmetal to a superconductor at about 160 gigapascals (GPa). The critical mination by a halogen lamp or Ar-ion laser (at temperature of the transition increases from 6 kelvin (K) at 175 GPa to 11.2 488 nm) as expected for -B, which is a semi- K at 250 GPa, giving a positive pressure derivative of 0.05 K/GPa. Although the conductor with a 1.6-eV energy gap (20). With observed metallization pressure is compatible with the predictions of first- increasing pressure at 300 K, a measurable re- principles calculations, superconductivity in boron remains to be explored sistance appeared above 19 GPa, and visible theoretically. -

Physics Reports a Perspective on Conventional High
Physics Reports 856 (2020) 1–78 Contents lists available at ScienceDirect Physics Reports journal homepage: www.elsevier.com/locate/physrep A perspective on conventional high-temperature superconductors at high pressure: Methods and materials José A. Flores-Livas a, Lilia Boeri a, Antonio Sanna b, Gianni Profeta c, ∗ Ryotaro Arita d,e, , Mikhail Eremets f a Department of Physics, Sapienza Universita' di Roma, Italy b Max-Planck Institute of Microstructure Physics, Weinberg 2, 06120 Halle, Germany c Department of Physical and Chemical Sciences and SPIN-CNR, University of L'Aquila, Via Vetoio 10, I-67100 L'Aquila, Italy d Department of Applied Physics, Hongo Bunkyo-ku 113-8656, Japan e RIKEN Center for Emergent Matter Science, 2-1 Hirosawa, Wako, 351-0198, Japan f Max-Planck Institute for Chemistry, Hahn-Meitner-Weg 1 55128 Mainz, Germany article info a b s t r a c t Article history: Two hydrogen-rich materials, H3S and LaH10, synthesized at megabar pressures, have Received 14 May 2019 revolutionized the field of condensed matter physics providing the first glimpse to Received in revised form 5 February 2020 the solution of the hundred-year-old problem of room temperature superconductivity. Accepted 6 February 2020 The mechanism underlying superconductivity in these exceptional compounds is the Available online 15 February 2020 conventional electron–phonon coupling. Here we describe recent advances in experi- Editor: N. Nagaosa mental techniques, superconductivity theory and first-principles computational methods Keywords: which have made possible these discoveries. This work aims to provide an up-to-date High-pressure chemistry compendium of the available results on superconducting hydrides and explain how the Hydrides synergy of different methodologies led to extraordinary discoveries in the field. -

Stability of Hypothetical Agiicl2 Polymorphs Under High Pressure
II Stability of hypothetical Ag Cl2 polymorphs under high pressure, revisited – a computational study Adam Grzelak1* and Wojciech Grochala1 1Center for New Technologies, University of Warsaw, Banacha 2C, 02-097 Warszawa, Poland *[email protected] Abstract A comparative computational study of stability of candidate structures for an as-yet unknown silver dichloride AgCl2 is presented. It is found that all considered candidates have a negative enthalpy of formation, but are unstable towards charge transfer and decomposition into silver(I) chloride and chlorine within the DFT and hybrid-DFT approaches in the entire studied pressure range. Within SCAN approach, II several of the “true” Ag Cl2 polymorphs (i.e. containing Ag(II) species) exhibit a region of stability below ca. 20 GPa. However, their stability with respect to aforementioned decomposition decreases with pressure by account of all three DFT methods, which suggests a limited possibility of high-pressure synthesis of AgCl2. Some common patterns in pressure-induced structural transitions observed in the studied systems also emerge, which further testify to an instability of hypothetical AgCl2 towards charge transfer and phase separation. Introduction Chemistry of silver(II) compounds constitutes a topic of studies that is both demanding – particularly due to the extremely strong oxidizing properties of these compounds – as well as interesting, as evidenced by the body of works discussing them in relation to oxocuprates – a well-known family of precursors for high-pressure superconductors. [1,2] Inspired by experimental works exploring high- pressure phase transitions of AgF2, [3,4] as well as most recent computational study of thermodynamic stability of hypothetical mixed-valence silver fluorides (including at elevated pressure conditions), [5] this work is a continuation to a previous systematic study, which explored relative stability of multiple II hypothetical polymorphs of Ag Cl2 – an as-yet unknown analogue of AgF2.