Durham E-Theses
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heteroptera: Gerromorpha) in Central Europe
Shortened web version University of South Bohemia in České Budějovice Faculty of Science Ecology of Veliidae and Mesoveliidae (Heteroptera: Gerromorpha) in Central Europe RNDr. Tomáš Ditrich Ph.D. Thesis Supervisor: Prof. RNDr. Miroslav Papáček, CSc. University of South Bohemia, Faculty of Education České Budějovice 2010 Shortened web version Ditrich, T., 2010: Ecology of Veliidae and Mesoveliidae (Heteroptera: Gerromorpha) in Central Europe. Ph.D. Thesis, in English. – 85 p., Faculty of Science, University of South Bohemia, České Budějovice, Czech Republic. Annotation Ecology of Veliidae and Mesoveliidae (Hemiptera: Heteroptera: Gerromorpha) was studied in selected European species. The research of these non-gerrid semiaquatic bugs was especially focused on voltinism, overwintering with physiological consequences and wing polymorphism with dispersal pattern. Hypotheses based on data from field surveys were tested by laboratory, mesocosm and field experiments. New data on life history traits and their ecophysiological consequences are discussed in seven original research papers (four papers published in peer-reviewed journals, one paper accepted to publication, one submitted paper and one communication in a conference proceedings), creating core of this thesis. Keywords Insects, semiaquatic bugs, life history, overwintering, voltinism, dispersion, wing polymorphism. Financial support This thesis was mainly supported by grant of The Ministry of Education, Youth and Sports of the Czech Republic No. MSM 6007665801, partially by grant of the Grant Agency of the University of South Bohemia No. GAJU 6/2007/P-PřF, by The Research Council of Norway: The YGGDRASIL mobility program No. 195759/V11 and by Czech Science Foundation grant No. 206/07/0269. Shortened web version Declaration I hereby declare that I worked out this Ph.D. -

191 Distribution of Gerris Asper and G. Lateralis (Hemiptera: Heteroptera
Published December 28, 2012 Klapalekiana, 48: 191–202, 2012 ISSN 1210-6100 Distribution of Gerris asper and G. lateralis (Hemiptera: Heteroptera: Gerridae) in the Czech Republic Rozšíření Gerris asper a G. lateralis (Hemiptera: Heteroptera: Gerridae) v České republice Petr JEZIORSKI1), Petr KMENT2), Tomáš DITRICH3), Michal STRAKA4), Jan SYCHRA4) & Libor Dvořák5) 1) Na Bělidle 1, CZ-735 64 Havířov-Suchá, Czech Republic; e-mail: [email protected] 2) Department of Entomology, National Museum, kunratice 1, CZ-148 00 Praha 4, Czech Republic; e-mail: [email protected] 3) Department of Biology, Faculty of Education, University of South Bohemia, Jeronýmova 10, CZ-371 15 České Budějovice, Czech Republic; e-mail: [email protected] 4) Department of Botany and Zoology, Faculty of Science, Masaryk University, kotlářská 2, CZ-611 37 Brno, Czech Republic; e-mails: [email protected], [email protected] 5) Municipal Museum Mariánské Lázně, Goethovo náměstí 11, CZ-353 01 Mariánské Láz- ně, Czech Republic; e-mail: [email protected] Heteroptera, Gerridae, Gerris asper, Gerris lateralis, distribution, new records, Czech Republic Abstract. All the available published and unpublished distributional records of two endangered pond skaters, Gerris asper (Fieber, 1860) and Gerris lateralis Schummel, 1832 (Hemiptera: Heteroptera: Gerridae) in the Czech Republic are summarized and mapped. Gerris asper was originally described from Bohemia by Fieber (1860) without any exact locality and we have been unable to confirm its occurrence with any subsequently collected specimen. The distribution of G. asper in the Czech Republic thus seems to be limited to southern and central Moravia, with a single new record from northern Moravia, where it was found syntopically with G. -
Water Bug ID Guide
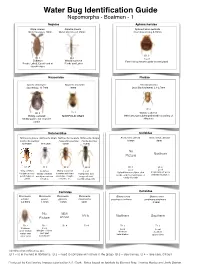
Water Bug Identification Guide Nepomorpha - Boatmen - 1 Nepidae Aphelocheridae Nepa cinerea Ranatra linearis Aphelocheirus aestivalis Water Scorpion, 20mm Water Stick Insect, 35mm River Saucer bug, 8-10mm ID 1 ID 1 ID 1 Local Common Widely scattered Fast flowing streams under stones/gravel Ponds, Lakes, Canals and at Ponds and Lakes stream edges Naucoridae Pleidae Ilycoris cimicoides Naucoris maculata Plea minutissima Saucer bug, 13.5mm 10mm Least Backswimmer, 2.1-2.7mm ID 1 ID 1 ID 2 Widely scattered Widely scattered NORFOLK ONLY Often amongst submerged weed in a variety of Muddy ponds and stagnant stillwaters canals Notonectidae Corixidae Notonecta glauca Notonecta viridis Notonecta maculata Notonecta obliqua Arctocorisa gemari Arctocorisa carinata Common Backswimmer Peppered Backswimmer Pied Backswimmer 8.8mm 9mm 14-16mm 13-15mm 15mm 15mm No Northern Picture ID 2 ID 2 ID 2 ID 2 ID 3 ID 3 Local Local Very common Common Widely scattered Local Upland limestone lakes, dew In upland peat pools Ubiquitous in all Variety of waters In waters with hard Peat ponds, acid ponds, acid moorland lakes, or with little vegetation ponds, lakes or usually more base substrates, troughs, bog pools and sandy silt ponds canals rich sites concrete, etc. recently clay ponds Corixidae Corixidae Micronecta Micronecta Micronecta Micronecta Glaenocorisa Glaenocorisa scholtzi poweri griseola minutissima propinqua cavifrons propinqua propinqua 2-2.5mm 1.8mm 1.8mm 2mm 8.3mm No NEW RARE Northern Southern Picture ARIVAL ID 2 ID 2 ID 4 ID 4 ID 3 ID 3 Local Common Local Local Margins of rivers open shallow Northern Southern and quiet waters over upland lakes upland lakes silt or sand backwaters Identification difficulties are: ID 1 = id in the field in Northants. -

The Relative Roles of Predation and Sperm Competition on the Duration of the Post-Copulatory Association Between the Sexes in the Blue Crab, Callinectes Sapidus
Behav Ecol Sociobiol (1997) 40: 175 – 185 Springer-Verlag 1997 Paul Jivoff The relative roles of predation and sperm competition on the duration of the post-copulatory association between the sexes in the blue crab, Callinectes sapidus Received: 23 January 1996 / Accepted after revision: 9 November 1996 Abstract In many species, post-copulatory mate were in the female spermathecae, another response to guarding prevents other males from mating with the sperm competition. Larger ejaculates may need longer guarded female. In crabs, males stay with their mates to post-copulatory associations before a more effective protect the female from predators because, in some sperm plug forms. Large males stayed with the female species, mating occurs when she is soft and vulnerable longer, which is consistent with their ability to pass after molting. I tested the relative roles of sperm com- larger ejaculates than small males and suggests that there petition and predation on the duration of the post- may be costs to minimizing the duration of the post- copulatory association in the blue crab, Callinectes copulatory association. In the field, associations last sapidus. Unpaired females suffered greater predation long enough to protect the female during her vulnerable mortality than paired females and males stayed with the phase and may ensure that the guarding male fertilizes female longer in the presence of predators than in their the most eggs in the female, even if she remates. Thus, absence, suggesting that the post-copulatory association the post-copulatory association protects female blue protects females during their vulnerable period. How- crabs from additional inseminators as well as from ever, the association may also occur in blue crabs be- predators. -

The Role of Mating Systems in Sexual Selection in Parasitoid Wasps
Biol. Rev. (2014), pp. 000–000. 1 doi: 10.1111/brv.12126 Beyond sex allocation: the role of mating systems in sexual selection in parasitoid wasps Rebecca A. Boulton∗, Laura A. Collins and David M. Shuker Centre for Biological Diversity, School of Biology, University of St Andrews, Dyers Brae, Greenside place, Fife KY16 9TH, U.K. ABSTRACT Despite the diverse array of mating systems and life histories which characterise the parasitic Hymenoptera, sexual selection and sexual conflict in this taxon have been somewhat overlooked. For instance, parasitoid mating systems have typically been studied in terms of how mating structure affects sex allocation. In the past decade, however, some studies have sought to address sexual selection in the parasitoid wasps more explicitly and found that, despite the lack of obvious secondary sexual traits, sexual selection has the potential to shape a range of aspects of parasitoid reproductive behaviour and ecology. Moreover, various characteristics fundamental to the parasitoid way of life may provide innovative new ways to investigate different processes of sexual selection. The overall aim of this review therefore is to re-examine parasitoid biology with sexual selection in mind, for both parasitoid biologists and also researchers interested in sexual selection and the evolution of mating systems more generally. We will consider aspects of particular relevance that have already been well studied including local mating structure, sex allocation and sperm depletion. We go on to review what we already know about sexual selection in the parasitoid wasps and highlight areas which may prove fruitful for further investigation. In particular, sperm depletion and the costs of inbreeding under chromosomal sex determination provide novel opportunities for testing the role of direct and indirect benefits for the evolution of mate choice. -

Spatial Distribution of Semiaquatic Bugs (Heteroptera: Gerromorpha
Silva Gabreta vol. 14 (3) p. 173–178 Vimperk, 2008 Spatial distribution of semiaquatic bugs (Heteroptera: Gerromorpha) and their wing morphs in a small scale of the Pohořský Potok stream spring area (Novohradské Hory Mts.) Tomáš Ditrich1,2,*, Miroslav Papáček1 & Tomáš Broum3 1Department of Biology, Pedagogical Faculty, University of South Bohemia, Jeronýmova 10, CZ-37115 České Budějovice, Czech Republic 2Department of Ecosystem Biology, Faculty of Science, University of South Bohemia, Branišovská 31, CZ-37005 České Budějovice, Czech Republic 3Větrná 603, CZ-43151 Klášterec nad Ohří, Czech Republic *[email protected] Abstract A survey of semiaquatic bugs (Heteroptera: Gerromorpha) was managed in a small scale area (72 ha; spring area of Pohořský Potok stream, Novohradské Hory Mts., Czech Republic, Central Europe). Species compo- sition of assemblages and rates of their pteromorphs were observed in the relation to selected environmen- tal characteristics – stream velocity, site permanence, water surface coverage and site shading. Gerris gibbifer Schummel, 1832, Gerris lateralis Schummel, 1832 and Velia caprai Tamanini, 1947 are dominant gerromorphan species in the study area. Gerris species are univoltine; bivoltinism of Veliidae species can- not be excluded in this area. A redundancy analysis showed significant effect of stream velocity, site shading and site permanence on species and wing morphs composition of assemblages. Velia caprai mostly occur in shaded habitats with flowing water, whereas other species prefer still water bodies. Gerris lateralis pre- fers shaded sites as the only gerrid. Occurrence of wing morphs of the only notably wing dimorphic speci- es G. lateralis in the study area depends on site permanence. Permanent water bodies are occupied by both macropterous and apterous specimens, temporary sites are almost exclusively colonized by macropterous individuals. -

423 – Aquatic Hemiptera of Northeastern
AQUATIC HEMIPTERA OF NORTHEASTERN ALGERIA: DISTRIBUTION, PHENOLOGY AND CONSERVATION Fouzi ANNANI 1,2, Ahmed H. AL F ARHAN 3 & Boudjéma SA M RAOUI 1,3* RÉSUMÉ.— Hémiptères aquatiques du nord-est de l’Algérie : distribution, phénologie et conserva- tion.— L’échantillonnage de 83 sites à travers le complexe de zones humides du nord-est Algérien, un point chaud de la biodiversité aquatique, a permis d’identifier 35 espèces d’hémiptères aquatiques. La répartition et la phénologie des espèces sont présentées et les histoires de vie de Notonecta glauca et Notonecta obli- qua déduites. Ces deux espèces estivent dans des milieux refuges à hautes altitudes avant de redescendre se reproduire en plaine à l’automne. Diverses manifestations de changements globaux (pompage de l’eau, construction de barrages, introduction d’espèces exotiques et fragmentation des milieux) influencent néga- tivement l’intégrité écologique des milieux de la région étudiée. SUMMARY.— A survey, involving the sampling of 83 sites, investigated the aquatic hemiptera of north- eastern Algeria, a well known hotspot of aquatic biodiversity. The study recorded 35 species with data on distribution and phenology presented and discussed. Aspects of the life history of some species (Notonecta glauca and Notonecta obliqua) were inferred from their distribution and phenology and they were found to aestivate at high altitude refuges. Insect conservation in North Africa is still embryonic, relying mainly on protected areas to provide surrogate conservation to a rich and diverse group. This is inadequate in view of the current distribution of aquatic insects, often located in unprotected habitats (intermittent streams, temporary pools, dunary ponds) and the fact that diverse manifestations of global changes (loss of habitats due to water extraction and dam construction, invasive species, habitat fragmentation) are fast eroding the biodiversity of protected areas. -

Het News Issue 3
Issue 3 Spring 2004 Het News nd 2 Series Newsletter of the Heteroptera Recording Schemes Editorial: There is a Dutch flavour to this issue which we hope will be of interest. After all, The Netherlands is not very far as the bug flies and with a following wind there could easily be immigrants reaching our shores at any time. We have also introduced an Archive section, for historical articles, to appear when space allows. As always we are very grateful to all the providers of material for this issue and, for the next issue, look forward to hearing about your 2004 (& 2003) exploits, exciting finds, regional news, innovative gadgets etc. Sheila Brooke 18 Park Hill Toddington Dunstable Beds LU5 6AW [email protected] Bernard Nau 15 Park Hill Toddington Dunstable Beds LU5 6AW [email protected] Contents Editorial .................................................................... 1 Forthcoming & recent events ................................. 7 Dutch Bug Atlas....................................................... 1 Checklist of British water bugs .............................. 8 Recent changes in the Dutch Heteroptera............. 2 The Lygus situation............................................... 11 Uncommon Heteroptera from S. England ............. 5 Web Focus.............................................................. 12 News from the Regions ........................................... 6 From the Archives ................................................. 12 Gadget corner – Bug Mailer..................................... -

The Freshwater Bugs (Hemiptera: Heteroptera) of Cheshire
The freshwater bugs (Hemiptera: Heteroptera) of Cheshire Jonathan Guest Germany. The late Dr. Alan Savage School of Life Sciences, Keele University, STS 5BG. Dr. Ian Wallace Entomology Section, Liverpool Museum, William Brown St., Liverpool, L3 8EN.* ______________________________ *address for correspondence_____________________ Introduction Cheshire is predominantly a lowland county. It has a very large number of ponds, internationally important habitats in the form of meres and mosses, intriguing inland saline waters associated with the salt industry and two major estuaries. Upland water bodies are restricted to the far east of the county. Eleven families of British Heteroptera are grouped as aquatic bugs with 64 species in 23 genera; Cheshire is both well-recorded and well-represented with 10 families, and 48 species in 21 genera. By stark contrast, the record coverage maps in Huxley (2003) show clearly that Lancashire, away from the coast, and especially south Lancashire, is very poorly recorded. Water bugs are very familiar freshwater insects, with most species being easy to catch in a pond-net; a few are best sought by pressing marginal vegetation underwater, and one lives amongst Sphagnum moss. They can be preserved in isopropyl alcohol, or a 70% ethyl alcohol solution - the traditional medium used by freshwater biologists, or pinned, or even carded - but important identification features are found on the underside in several genera. Identification is straightforward and satisfying using Savage (1989) for adults; for immatures of the large family Corixidae see Savage (1999) and for young pondskaters there is Brinkhurst (1959). Information sources Britten (1930) provided the first check-list for Cheshire water bugs, which was updated by Massee (1955) and Judd (1990). -

Male and Female Genotype and a Genotype-By-Genotype Interaction Mediate the Effects of Mating on Cellular but Not Humoral Immunity in Female Decorated Crickets
Heredity (2021) 126:477–490 https://doi.org/10.1038/s41437-020-00384-8 ARTICLE Male and female genotype and a genotype-by-genotype interaction mediate the effects of mating on cellular but not humoral immunity in female decorated crickets 1 1 2,3 1 1 Kylie J. Hampton ● Kristin R. Duffield ● John Hunt ● Scott K. Sakaluk ● Ben M. Sadd Received: 17 April 2020 / Revised: 22 October 2020 / Accepted: 26 October 2020 / Published online: 20 November 2020 © The Author(s), under exclusive licence to The Genetics Society 2020 Abstract Sexually antagonistic coevolution is predicted to lead to the divergence of male and female genotypes related to the effects of substances transferred by males at mating on female physiology. The outcome of mating should thus depend on the specific combination of mating genotypes. Although mating has been shown to influence female immunity in diverse insect taxa, a male–female genotype-by-genotype effect on female immunity post mating remains largely unexplored. Here, we investigate the effects of mating on female decorated cricket baseline immunity and the potential for a male-genotype-by-female-genotype interaction affecting this response. Females from three distinct genotypic backgrounds were left unmated or singly mated in a 1234567890();,: 1234567890();,: fully reciprocal design to males from the same three genotypic backgrounds. Hemocytes and hemocyte microaggregations were quantified for female cellular immunity, and phenoloxidase, involved in melanization, and antibacterial activity for humoral immunity. In this system, female cellular immunity was more reactive to mating, and mating effects were genotype-dependent. Specifically, for hemocytes, a genotype-by-mating status interaction mediated the effect of mating per se, and a significant male–female genotype-by-genotype interaction determined hemocyte depletion post mating. -

Taxaliste Der Gewässerorganismen Deutschlands Zur Kodierung
Bayerisches Landesamt für Wasserwirtschaft (Herausgeber und Verlag) · München 2003 Taxaliste der Gewässer- organismen Deutschlands zur Kodierung biologischer Befunde Informationsberichte Heft 1/03 Informationsberichte des Bayerischen Landsamtes für Wasserwirtschaft Heft 01/03 München, 2003 – ISBN 3-930253-89-5 367 Seiten, 6 Abbildungen, 1 Tabelle, 1 CD, gedruckt auf chlorfrei gebleichtem Papier Herausgeber: Bayerisches Landesamt für Wasserwirtschaft, Lazarettstraße 67, D-80636 München, eine Behörde im Geschäftsbereich des Bayerischen Staatsministeriums für Landesentwicklung und Umweltfragen Autoren: Dr. Erik Mauch Dr. Ursula Schmedtje Dipl.-Ing (FH) Anette Maetze Dipl.-Biol. Folker Fischer Druck: Druckhaus Fritz König GmbH, München Bezug: Wasserwirtschaftsamt Deggendorf, Postfach 2061, 94460 Deggendorf Nachdruck und Wiedergabe – auch auszugsweise – nur mit Genehmigung des Herausgebers Vorwort Für die nationale und internationale Zusammenarbeit im Bereich der biologischen Gewässer- untersuchung ist die eindeutige Benennung und Kodierung der Gewässerorganismen entschei- dend. Diesem Zweck dient die „Taxaliste der Gewässerorganismen Deutschlands“, die hiermit erstmalig veröffentlicht wird. Die Liste enthält fast 10 000 in Deutschland vorkommende Gewässerorganismen der unterschied- lichsten limnischen Lebensräume. Jedem dieser Taxa wurde eine eindeutige Kennziffer zugewiesen. So ist es möglich, die Untersuchungsergebnisse innerhalb sowie zwischen den Flussgebieten in einheitlichen Datenbanken zusammenzufassen und auszuwerten. Damit stellt -
Long-Term Trends and Drivers of Aquatic Insects in the Netherlands
LONG-TERM TRENDS AND DRIVERS OF AQUATIC INSECTS IN THE NETHERLANDS 2021 39 1 1 3 LONG-TERM TRENDS AND DRIVERS OF AQUATIC INSECTS IN THE NETHERLANDS CASPAR A. HALLMANN EELKE JONGEJANS 2021 39 PB 1 Water beetle - Cybister lateralimarginalis - tuimelaar Photo: Bureau Biota COLOPHON STOWA-number 2021-39 ISBN 978.90.5773.943.9 Download This report is available as pdf on www.stowa.nl Check Bibliotheek > Publicaties > STOWA 2021-39 Publication STOWA | P.O. Box 2180 | 3800 CD Amersfoort August 2021 © STOWA 2 Plecoptera Nemoura cinerea larvae Photo:Bureau Biota Authors Design Caspar A. Hallmann1 & Eelke Jongejans1,18 Shapeshifter.nl | Utrecht In cooperation with Photography cover Ineke Barten6, Harry Boonstra14, Theo Cuijpers17, Hans de Kroon1, Ben Eenkhoorn11, Bureau Biota Boris Everwijn3, Ronald Gylstra15, Bram Koese2, John P.M. Lensen7, Mieke Moeleker12, Left: bush-tailed caddisfly - Sericostoma personatum - kokerjuffer Astra Ooms11, Leo Posthuma1,8, Jaap Postma9, Ernst Raaphorst10, Dorien Roubos13, Right: common darter - Sympetrum striolatum - bruinrode heidelibel Carlo Rutjes5, Mark Scheepens6, Henk Siepel1, Jaap Slootweg8, Wilco C.E.P. Verberk1, Bas van der Wal3, Gert van Ee11, Barend van Maanen4, Tessa van Vreeswijk16, Copyright Danneke Verhagen-Bakker16 & Theo Zeegers2 The information in this publication may be used, provided the source is acknowledged. The knowledge in this report, developed or accumulated, is available free of charge. Costs 1 Radboud University, Radboud Institute for Biological and Environmental Sciences, STOWA charges for publications, are only costs for design, printing and shipping. Nijmegen, The Netherlands 2 EIS Kenniscentrum Insecten, Leiden, The Netherlands Disclaimer 3 STOWA Foundation for Applied Water Research, Amersfoort, The Netherlands This report is based on current insights and has been put together with care.