Tissue and Renal Response to Chronic Respiratory Acidosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pathophysiology of Acid Base Balance: the Theory Practice Relationship
Intensive and Critical Care Nursing (2008) 24, 28—40 ORIGINAL ARTICLE Pathophysiology of acid base balance: The theory practice relationship Sharon L. Edwards ∗ Buckinghamshire Chilterns University College, Chalfont Campus, Newland Park, Gorelands Lane, Chalfont St. Giles, Buckinghamshire HP8 4AD, United Kingdom Accepted 13 May 2007 KEYWORDS Summary There are many disorders/diseases that lead to changes in acid base Acid base balance; balance. These conditions are not rare or uncommon in clinical practice, but every- Arterial blood gases; day occurrences on the ward or in critical care. Conditions such as asthma, chronic Acidosis; obstructive pulmonary disease (bronchitis or emphasaemia), diabetic ketoacidosis, Alkalosis renal disease or failure, any type of shock (sepsis, anaphylaxsis, neurogenic, cardio- genic, hypovolaemia), stress or anxiety which can lead to hyperventilation, and some drugs (sedatives, opoids) leading to reduced ventilation. In addition, some symptoms of disease can cause vomiting and diarrhoea, which effects acid base balance. It is imperative that critical care nurses are aware of changes that occur in relation to altered physiology, leading to an understanding of the changes in patients’ condition that are observed, and why the administration of some immediate therapies such as oxygen is imperative. © 2007 Elsevier Ltd. All rights reserved. Introduction the essential concepts of acid base physiology is necessary so that quick and correct diagnosis can The implications for practice with regards to be determined and appropriate treatment imple- acid base physiology are separated into respi- mented. ratory acidosis and alkalosis, metabolic acidosis The homeostatic imbalances of acid base are and alkalosis, observed in patients with differing examined as the body attempts to maintain pH bal- aetiologies. -

./0) (Mm...)Conte H
BRITISH 511 Auc,.Auo. 25,25, 19621962 CARBON-MONOXIDE POISONIN6 MEDICAL JOURNAL Br Med J: first published as 10.1136/bmj.2.5303.511 on 25 August 1962. Downloaded from Case Reports HYPERVENTILATION IN Case 1.-A woman aged 61 was found with her head in CARBON-MONOXIDE POISONING a gas-oven. On admission to hospital she was deeply unconscious, with a generalized increase of muscle tone. BY pulse rate 140 a minute, and blood-pressure 110/70 mm. Hg. G. L. LEATHART, M.D., M.R.C.P. There was marked hyperventilation suggesting the possibility of coincident aspirin poisoning. A stomach wash-out, how- Nuflield Department of Industrial Health, the Medical ever, revealed no tablets, and a sample of urine collected School, King's College, Newcastle upon Tyne a few hours later contained no detectable salicylate. In 24 hours she had recovered fully and was transferred to a The recent revival of interest in the use of 5% or 7% mental hospital. carbogen in the treatment of carbon-monoxide poisoning Case 2.-An accountant aged 40 was working late in his has prompted the description of four unusual cases in office and was seen to be well at 9.15 p.m. At 8.45 a.m. which gross hyperventilation occurred. The investiga- the following morning he was found in the office with the tion of these cases was not very thorough, but such cases gas turned on but unlit. He was deeply unconscious with are seen so seldom that it is felt that even this incomplete strikingly deep and rapid respiration suggesting a condition report may be of value in stimulating further research. -

TITLE: Acid-Base Disorders PRESENTER: Brenda Suh-Lailam
TITLE: Acid-Base Disorders PRESENTER: Brenda Suh-Lailam Slide 1: Hello, my name is Brenda Suh-Lailam. I am an Assistant Director of Clinical Chemistry and Mass Spectrometry at Ann & Robert H. Lurie Children’s Hospital of Chicago, and an Assistant Professor of Pathology at Northwestern Feinberg School of Medicine. Welcome to this Pearl of Laboratory Medicine on “Acid-Base Disorders.” Slide 2: During metabolism, the body produces hydrogen ions which affect metabolic processes if concentration is not regulated. To maintain pH within physiologic limits, there are several buffer systems that help regulate hydrogen ion concentration. For example, bicarbonate, plasma proteins, and hemoglobin buffer systems. The bicarbonate buffer system is the major buffer system in the blood. Slide 3: In the bicarbonate buffer system, bicarbonate, which is the metabolic component, is controlled by the kidneys. Carbon dioxide is the respiratory component and is controlled by the lungs. Changes in the respiratory and metabolic components, as depicted here, can lead to a decrease in pH termed acidosis, or an increase in pH termed alkalosis. Slide 4: Because the bicarbonate buffer system is the major buffer system of blood, estimation of pH using the Henderson-Hasselbalch equation is usually performed, expressed as a ratio of bicarbonate and carbon dioxide. Where pKa is the pH at which the concentration of protonated and unprotonated species are equal, and 0.0307 is the solubility coefficient of carbon dioxide. Four variables are present in this equation; knowing three variables allows for calculation of the fourth. Since pKa is a constant, and pH and carbon dioxide are measured during blood gas analysis, bicarbonate can, therefore, be determined using this equation. -

Important Prescribing Information
Important Prescribing Information Subject: Temporary importation of 8.4% Sodium Bicarbonate Injection to address drug shortage issues June 14, 2019 Dear Healthcare Professional, Due to the current critical shortage of Sodium Bicarbonate Injection, USP in the United States (US) market, Athenex Pharmaceutical Division, LLC (Athenex) is coordinating with the U.S. Food and Drug Administration (FDA) to increase the availability of Sodium Bicarbonate Injection. Athenex has initiated temporary importation of another manufacturer’s 8.4% Sodium Bicarbonate Injection (1 mEq/mL) into the U.S. market. This product is manufactured and marketed in Australia by Phebra Pty Ltd (Phebra). At this time, no other entity except Athenex Pharmaceutical Division, LLC is authorized by the FDA to import or distribute Phebra’s 8.4% Sodium Bicarbonate Injection, (1 mEq/mL), 10 mL vials, in the United States. FDA has not approved Phebra’s 8.4% Sodium Bicarbonate Injection but does not object to its importation into the United States. Effective immediately, and during this temporary period, Athenex will offer the following presentation of Sodium Bicarbonate Injection: Sodium Bicarbonate Injection, 8.4% (1mEq/mL), 10mL per vial, 10 vials per carton Ingredients: sodium bicarbonate, water for injection, disodium edetate and sodium hydroxide (pH adjustment) Marketing Authorization Number in Australia is: 131067 Phebra’s Sodium Bicarbonate Injection contains the same active ingredient, Sodium Bicarbonate, in the same strength and concentration, 8.4% (1 mEq/mL) as the U.S. registered Sodium Bicarbonate Injection, USP by Pfizer’s subsidiary, Hospira. However, it is important to note that Phebra’s Sodium Bicarbonate Injection (1 mEq/mL), is provided only in a Single Use 10 mL vials, whereas Hospira’s product is provided in 50 mL single-dose vials and syringes. -

Parenteral Nutrition Primer: Balance Acid-Base, Fluid and Electrolytes
Parenteral Nutrition Primer: Balancing Acid-Base, Fluids and Electrolytes Phil Ayers, PharmD, BCNSP, FASHP Todd W. Canada, PharmD, BCNSP, FASHP, FTSHP Michael Kraft, PharmD, BCNSP Gordon S. Sacks, Pharm.D., BCNSP, FCCP Disclosure . The program chair and presenters for this continuing education activity have reported no relevant financial relationships, except: . Phil Ayers - ASPEN: Board Member/Advisory Panel; B Braun: Consultant; Baxter: Consultant; Fresenius Kabi: Consultant; Janssen: Consultant; Mallinckrodt: Consultant . Todd Canada - Fresenius Kabi: Board Member/Advisory Panel, Consultant, Speaker's Bureau • Michael Kraft - Rockwell Medical: Consultant; Fresenius Kabi: Advisory Board; B. Braun: Advisory Board; Takeda Pharmaceuticals: Speaker’s Bureau (spouse) . Gordon Sacks - Grant Support: Fresenius Kabi Sodium Disorders and Fluid Balance Gordon S. Sacks, Pharm.D., BCNSP Professor and Department Head Department of Pharmacy Practice Harrison School of Pharmacy Auburn University Learning Objectives Upon completion of this session, the learner will be able to: 1. Differentiate between hypovolemic, euvolemic, and hypervolemic hyponatremia 2. Recommend appropriate changes in nutrition support formulations when hyponatremia occurs 3. Identify drug-induced causes of hypo- and hypernatremia No sodium for you! Presentation Outline . Overview of sodium and water . Dehydration vs. Volume Depletion . Water requirements & Equations . Hyponatremia • Hypotonic o Hypovolemic o Euvolemic o Hypervolemic . Hypernatremia • Hypovolemic • Euvolemic • Hypervolemic Sodium and Fluid Balance . Helpful hint: total body sodium determines volume status, not sodium status . Examples of this concept • Hypervolemic – too much volume • Hypovolemic – too little volume • Euvolemic – normal volume Water Distribution . Total body water content varies from 50-70% of body weight • Dependent on lean body mass: fat ratio o Fat water content is ~10% compared to ~75% for muscle mass . -

Rational Treatment of Acid-Base Disorders
Practical Therapeutics Drugs 39 (6): 841-855, 1990 0012-6667/90/0006-0841/$07.50/0 © ADlS Press Limited All rights reserved. DRUG03353 Rational Treatment of Acid-Base Disorders Margaret L. McLaughlin and Jerome P. Kassirer Nephrology Division, Department of Medicine, New England Medical Center, and Department of Med icine, Tufts Un iversity School of Medi cine, Boston , Massachusetts, USA Contents Summary , ,.., , ,.., ,.., ,..,.. 842 I. Metabolic Acidosis 843 1.1 Clinical Manifestations 843 1.2 Causes of Metabolic Acidosis 843 1.3 Treatment of Metabolic Acidosis 844 1.3.1 General Remarks 844 1.3.2 Loss of Alkaline Gastrointestinal Fluids 845 1.3.3 Carbonic Anhydrase Inhibitors 845 1.3.4 Urinary Diversion , 845 1.3.5 Lactic Acidosis 845 1.3.6 Diabetic Ketoacidosis 846 1.3.7 Alcoholic Ketoacidosis 846 1.3.8 Renal Tubular Acidosis ll47 1.3.9 Renal Acidosis (Uraemic Acidosis) 848 2. Metabolic Alkalosis , 848 2.1 Clinical Manifestations 848 2.2 Causes of Metabolic Alkalosis 848 2.3 Treatment of Metabolic Alkalosis 849 2.3.1 Milk-Alkali Syndrome 850 2.3.2 Combined Therapy with Nonabsorbabl e Alkali and Exchange Resins 850 2.3.3 Acute Alkali Loading 850 2.3.4 Gastric Fluid Losses 850 2.3.5 Diuretic Therapy , 851 2.3.6 Posthypercapnic Alkalosis 851 2.3.7 Primary Aldosteron ism 851 2.3.8 Bartter's Syndrom e 85I 2.3.9 Cushing's Syndrome 852 3. Respiratory Acidosis 852 3.1 Clinical Manifestations 852 3.2 Causes of Respiratory Acidosis , 852 3.3 Treatment of Respiratory Acidosis , 852 4. -

Metabolic Alkalosis in Adults with Stable Cystic Fibrosis Fahad Al-Ghimlas*,1, Marie E
The Open Respiratory Medicine Journal, 2012, 6, 59-62 59 Open Access Metabolic Alkalosis in Adults with Stable Cystic Fibrosis Fahad Al-Ghimlas*,1, Marie E. Faughnan1,2 and Elizabeth Tullis1,2 1Department of Medicine, University of Toronto, Canada 2St. Michael’s Hospital, Li Ka Shing Knowledge Institute, Canada Abstract: Background: The frequency of metabolic alkalosis among adults with stable severe CF-lung disease is unknown. Methods: Retrospective chart review. Results: Fourteen CF and 6 COPD (controls) patients were included. FEV1 was similar between the two groups. PaO2 was significantly higher in the COPD (mean ± 2 SD is 72.0 ± 6.8 mmHg,) than in the CF group (56.1 ± 4.1 mmHg). The frequency of metabolic alkalosis in CF patients (12/14, 86%) was significantly greater (p=0.04) than in the COPD group (2/6, 33%). Mixed respiratory acidosis and metabolic alkalosis was evident in 4 CF and 1 COPD patients. Primary metabolic alkalosis was observed in 8 CF and none of the COPD patients. One COPD patient had respiratory and metabolic alkalosis. Conclusions: Metabolic alkalosis is more frequent in stable patients with CF lung disease than in COPD patients. This might be due to defective CFTR function with abnormal electrolyte transport within the kidney and/ or gastrointestinal tract. Keywords: Cystic fibrosis, metabolic alkalosis. BACKGROUND METHODS Cystic fibrosis (CF) is a multi-system disease that is After obtaining ethics approval, a retrospective chart and caused by mutations in a 230 kb gene on chromosome 7 database review was performed on clinically stable CF encoding 1480 aminoacid polypeptide, named cystic fibrosis patients (Toronto CF Database) and COPD patients (Chest transmembrane regulator (CFTR) [1]. -

Neurologic Complications of Electrolyte Disturbances and Acid–Base Balance
Handbook of Clinical Neurology, Vol. 119 (3rd series) Neurologic Aspects of Systemic Disease Part I Jose Biller and Jose M. Ferro, Editors © 2014 Elsevier B.V. All rights reserved Chapter 23 Neurologic complications of electrolyte disturbances and acid–base balance ALBERTO J. ESPAY* James J. and Joan A. Gardner Center for Parkinson’s Disease and Movement Disorders, Department of Neurology, UC Neuroscience Institute, University of Cincinnati, Cincinnati, OH, USA INTRODUCTION hyperglycemia or mannitol intake, when plasma osmolal- ity is high (hypertonic) due to the presence of either of The complex interplay between respiratory and renal these osmotically active substances (Weisberg, 1989; function is at the center of the electrolytic and acid-based Lippi and Aloe, 2010). True or hypotonic hyponatremia environment in which the central and peripheral nervous is always due to a relative excess of water compared to systems function. Neurological manifestations are sodium, and can occur in the setting of hypovolemia, accompaniments of all electrolytic and acid–base distur- euvolemia, and hypervolemia (Table 23.2), invariably bances once certain thresholds are reached (Riggs, reflecting an abnormal relationship between water and 2002). This chapter reviews the major changes resulting sodium, whereby the former is retained at a rate faster alterations in the plasma concentration of sodium, from than the latter (Milionis et al., 2002). Homeostatic mech- potassium, calcium, magnesium, and phosphorus as well anisms protecting against changes in volume and sodium as from acidemia and alkalemia (Table 23.1). concentration include sympathetic activity, the renin– angiotensin–aldosterone system, which cause resorption HYPONATREMIA of sodium by the kidneys, and the hypothalamic arginine vasopressin, also known as antidiuretic hormone (ADH), History and terminology which prompts resorption of water (Eiskjaer et al., 1991). -

ACS/ASE Medical Student Core Curriculum Acid-Base Balance
ACS/ASE Medical Student Core Curriculum Acid-Base Balance ACID-BASE BALANCE Epidemiology/Pathophysiology Understanding the physiology of acid-base homeostasis is important to the surgeon. The two acid-base buffer systems in the human body are the metabolic system (kidneys) and the respiratory system (lungs). The simultaneous equilibrium reactions that take place to maintain normal acid-base balance are: H" HCO* ↔ H CO ↔ H O l CO g To classify the type of disturbance, a blood gas (preferably arterial) and basic metabolic panel must be obtained. A basic understanding of normal acid-base buffer physiology is required to understand alterations in these labs. The normal pH of human blood is 7.40 (7.35-7.45). This number is tightly regulated by the two buffer systems mentioned above. The lungs contain carbonic anhydrase which is capable of converting carbonic acid to water and CO2. The respiratory response results in an alteration to ventilation which allows acid to be retained or expelled as CO2. Therefore, bradypnea will result in respiratory acidosis while tachypnea will result in respiratory alkalosis. The respiratory buffer system is fast acting, resulting in respiratory compensation within 30 minutes and taking approximately 12 to 24 hours to reach equilibrium. The renal metabolic response results in alterations in bicarbonate excretion. This system is more time consuming and can typically takes at least three to five days to reach equilibrium. Five primary classifications of acid-base imbalance: • Metabolic acidosis • Metabolic alkalosis • Respiratory acidosis • Respiratory alkalosis • Mixed acid-base disturbance It is important to remember that more than one of the above processes can be present in a patient at any given time. -

The ABC's of Acid-Base Balance
JPPT REVIEW ARTICLE The ABC’s of Acid-Base Balance Gordon S. Sacks, PharmD The University of Wisconsin—Madison, Madison, Wisconsin A step-wise systematic approach can be used to determine the etiology and proper management of acid-base disorders. The objectives of this article are to: (1) discuss the physiologic processes in- volved in acid-base disturbances, (2) identify primary and secondary acid-base disturbances based upon arterial blood gas and laboratory measurements, (3) utilize the anion gap for diagnostic pur- poses, and (4) outline a stepwise approach for interpretation and treatment of acid-base disorders. Case studies are used to illustrate the application of the discussed systematic approach. KEYWORDS: acid-base J Pediatr Pharmacol Ther 2004;9:235-42 Although acid-base disorders are frequently terms of H+, but due to confusing terminology it encountered in hospital and ambulatory care set- was proposed to convert H+ terminology to pH.1 tings, they are often considered the most difficult When taking the negative logarithm of the H+ areas to understand in medicine. Misdiagnosis due to common misconceptions of acid-base ho- ABBREVIATIONS: AG, Anion gap; HCO3, Bicarbonate; CNS, meostasis often delays identification of the pri- Central nervous system; ECF, Extracellular fluid; Hgb, Hemoglobin; ICU, Intensive care unit; THAM, Tromethamine mary disorder, causing a disruption in the deliv- ery of appropriate therapy. By understanding the concentration, pH represents a measure of H+ basic principles of acid-base physiology, the inter- activity. Optimal function for tissues and organs pretation of acid-base data, and the mechanisms within the human body depends on maintaining responsible for acid-base perturbations, the clini- blood pH between 7.10 and 7.60. -

Renal-Tubular-Acidosis-NAGMA
Non-Anion Gap Metabolic Acidosis App.GoSoapbox.com 665-971-584 then “Join Now” Joel M. Topf, M.D. http://PBFluids.com @kidney_boy App.GoSoapbox.com 665-971-584 32 y.o. female with fatigue, weakness and muscle aches App.GoSoapbox.com 665-971-584 Answer polls: • Uhura: What is the primary acid base… • Uhura: What is the appropriate pCO2… 139 115 16 7.34 / 33 / 87 / 18 3.1 17 1.0 32 y.o. female with fatigue, weakness and muscle aches 139 115 16 7.34 / 33 / 87 / 18 3.1 17 1.0 Determine the primary disorder 7.34 / 87 / 33 / 16 pH / pO2 / pCO2 / HCO3 1. Acidosis or alkalosis – If thethe pHpH is is less less than than 7.4 7.4 it it is is acidosis acidosis – If the pH is greater than 7.4 it is alkalosis 2. DetermineMetabolic if it is respiratory or metabolic Acidosis – If thethe pH,pH, bicarbonate bicarbonate and and pCO pCO2 all2 allmove move in thein thesame same direction direction (up or(up down)or down) it is it metabolic is metabolic – If the pH, bicarbonate and pCO2 move in discordant directions (up and down)and down) it is respiratoryit is respiratory Predicting pCO2 in metabolic acidosis: Winter’s Formula • In metabolic acidosis the expected pCO2 can be estimated from the HCO3 Expected pCO2 = (1.5 x HCO3) + 8 ± 2 • If the pCO2 is higher than predicted then there is an addition respiratory acidosis • If the pCO2 is lower than predicted there is an additional respiratory alkalosis Is the compensation appropriate? 7.33 / 87 / 33 / 17 pH / pO2 / pCO2 / HCO3 ± Appropriately• Expected pCO2 = (1.5 x HCOcompensated3) + 8 2 • Expected pCO2 = 31-35 metabolic acidosis • Actual pCO2 is 33, which is within the predicted range, indicating appropriate respiratory compensation What anion is associated with the additional acid? • Metabolic acidosis is further evaluated by determining the anion associated with the increased H+ cation • In medicine we categorize the anions into: chloride not chloride Non-Anion Gap Anion Gap Metabolic Acidosis Metabolic Acidosis …and differentiate the two based on the anion gap. -
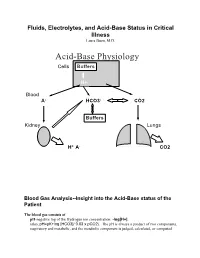
Acid-Base Physiology Cells Buffers
Fluids, Electrolytes, and Acid-Base Status in Critical Illness Laura Ibsen, M.D. Acid-Base Physiology Cells Buffers H+ Blood A- HCO3- CO2 Buffers Kidney Lungs H+ A- CO2 Blood Gas Analysis--Insight into the Acid-Base status of the Patient The blood gas consists of pH-negative log of the Hydrogen ion concentration: -log[H+]. (also, pH=pK+log [HCO3]/ 0.03 x pCO2). The pH is always a product of two components, respiratory and metabolic, and the metabolic component is judged, calculated, or computed by allowing for the effect of the pCO2, ie, any change in the pH unexplained by the pCO2 indicates a metabolic abnormality. - + CO2+H20º H2CO3ºHCO3 + H CO2 and water form carbonic acid or H2CO3, which is in equilibrium with bicarbonate (HCO3-)and hydrogen ions (H+). A change in the concentration of the reactants on either side of the equation affects the subsequent direction of the reaction. For example, an increase in CO2 will result in increased carbonic acid formation (H2CO3) which leads to an increase in both HCO3- and H+ (\pH). Normally, at pH 7.4, a ratio of one part carbonic acid to twenty parts bicarbonate is present in the extracellular fluid [HCO3-/H2CO3]=20. A change in the ratio will affect the pH of the fluid. If both components change (ie, with chronic compensation), the pH may be normal, but the other components will not. pCO2-partial pressure of carbon dioxide. Hypoventilation or hyperventilation (ie, minute ventilation--tidal volume x respitatory rate--imperfectly matched to physiologic demands) will lead to elevation or depression, respectively, in the pCO2.