Mechanisms of Song Perception in Oscine Birds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Auditory Experience Controls the Maturation of Song Discrimination and Sexual Response in Drosophila Xiaodong Li, Hiroshi Ishimoto, Azusa Kamikouchi*
RESEARCH ARTICLE Auditory experience controls the maturation of song discrimination and sexual response in Drosophila Xiaodong Li, Hiroshi Ishimoto, Azusa Kamikouchi* Graduate School of Science, Nagoya University, Nagoya, Japan Abstract In birds and higher mammals, auditory experience during development is critical to discriminate sound patterns in adulthood. However, the neural and molecular nature of this acquired ability remains elusive. In fruit flies, acoustic perception has been thought to be innate. Here we report, surprisingly, that auditory experience of a species-specific courtship song in developing Drosophila shapes adult song perception and resultant sexual behavior. Preferences in the song-response behaviors of both males and females were tuned by social acoustic exposure during development. We examined the molecular and cellular determinants of this social acoustic learning and found that GABA signaling acting on the GABAA receptor Rdl in the pC1 neurons, the integration node for courtship stimuli, regulated auditory tuning and sexual behavior. These findings demonstrate that maturation of auditory perception in flies is unexpectedly plastic and is acquired socially, providing a model to investigate how song learning regulates mating preference in insects. DOI: https://doi.org/10.7554/eLife.34348.001 Introduction Vocal learning in infants or juvenile birds relies heavily on the early experience of the adult conspe- cific sounds. In humans, early language input is necessary to form the ability of phonetic distinction *For correspondence: and pattern detection in the phase of auditory learning (Doupe and Kuhl, 1999; Kuhl, 2004). [email protected] Because of the strong parallels between speech acquisition of humans and song learning of song- Competing interests: The birds, and the difficulties to investigate the neural mechanisms of human early auditory memory at authors declare that no cellular resolution, songbirds have been used as a predominant model in studying memory formation competing interests exist. -

Multimodal Gaze Stabilization of a Humanoid Robot Based on Reafferences
Multimodal Gaze Stabilization of a Humanoid Robot based on Reafferences Timothee´ Habra1, Markus Grotz2, David Sippel2, Tamim Asfour2 and Renaud Ronsse1 Abstract— Gaze stabilization is fundamental for humanoid robots. By stabilizing vision, it enhances perception of the environment and keeps regions of interest inside the field of view. In this contribution, a multimodal gaze stabilization combining proprioceptive, inertial and visual cues is introduced. It integrates a classical inverse kinematic control with vestibulo- ocular and optokinetic reflexes. Inspired by neuroscience, our contribution implements a forward internal model that mod- ulates the reflexes based on the reafference principle. This principle filters self-generated movements out of the reflexive feedback loop. The versatility and effectiveness of this method are experimentally validated on the ARMAR-III humanoid robot. We first demonstrate that all the stabilization mech- (A) (B) anisms (inverse kinematics and reflexes) are complementary. Then, we show that our multimodal method, combining these Fig. 1. The ARMAR-III humanoid robot. (A) The robot in its home three modalities with the reafference principle, provides a versa- environment used to validate the gaze stabilization. The robot’s point of view is shown in the top left corner. (B) Kinematic chain of the head, with tile gaze stabilizer able to handle a large panel of perturbations. 4 degrees of freedom for the neck and 3 for the eyes. I. INTRODUCTION Vision plays a central role in our perception of the world. It the non-linear dynamics of the oculomotor system. Similarly, allows to interpret our surrounding environment at a glance. Lenz et al. introduced an adaptive VOR controller learning Not surprisingly, humanoid robots heavily rely on visual the oculomotor dynamics, but based on decorrelation control perception. -
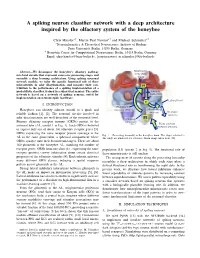
A Spiking Neuron Classifier Network with a Deep Architecture Inspired By
A spiking neuron classifier network with a deep architecture inspired by the olfactory system of the honeybee Chris Hausler¨ ∗†, Martin Paul Nawrot∗† and Michael Schmuker∗† ∗Neuroinformatics & Theoretical Neuroscience, Institute of Biology Freie Universitat¨ Berlin, 14195 Berlin, Germany † Bernstein Center for Computational Neuroscience Berlin, 10115 Berlin, Germany Email: [email protected], fmartin.nawrot, [email protected] Abstract—We decompose the honeybee’s olfactory pathway Mushroom into local circuits that represent successive processing stages and body (MB) calyx resemble a deep learning architecture. Using spiking neuronal network models, we infer the specific functional role of these microcircuits in odor discrimination, and measure their con- 2 tribution to the performance of a spiking implementation of a probabilistic classifier, trained in a supervised manner. The entire Kenyon network is based on a network of spiking neurons, suited for Cells (KC) implementation on neuromorphic hardware. Lateral horn 3 I. INTRODUCTION Projection Honeybees can identify odorant stimuli in a quick and neurons (PN) MB-extrinsic neurons To motor reliable fashion [1], [2]. The neuronal circuits involved in neurons odor discrimination are well described at the structural level. 1 Antennal Primary olfactory receptor neurons (ORNs) project to the lobe (AL) From odorant antennal lobe (AL, circuit 1 in Fig. 1). Each ORN is believed receptor neurons to express only one of about 160 olfactory receptor genes [3]. ORNs expressing the same receptor protein converge in the Fig. 1. Processing hierarchy in the honeybee brain. The stages relevant to AL in the same glomerulus, a spherical compartment where this study are numbered for reference. -

1P MON. PM Cortex and Subcortical Auditory Nuclei
dynamic role for the vestibular system in orientation and flight control. Laboratory studies of flight behavior under illuminated and dark conditions in both static and rotating obstacle tests were carried out while administering heavy water ͑D2O͒ to bats to impair their vestibular inputs. Eptesicus carried out complex maneuvers through both fixed arrays of wires and a rotating obstacle array using both vision and echolocation, or when guided by echolocation alone. When treated with D2O in combination with lack of visual cues, bats showed considerable decrements in performance. These data indicate that big brown bats use both vision and echolocation to provide spatial registration for head position information generated by the vestibular system. 2:55 1pAB3. Bat’s auditory system: Corticofugal feedback and plasticity. Nobuo Suga ͑Dept. of Biol., Washington Univ., One Brookings Dr., St. Louis, MO 63130͒ The auditory system of the mustached bat consists of physiologically distinct subdivisions for processing different types of biosonar information. It was found that the corticofugal ͑descending͒ auditory system plays an important role in improving and adjusting auditory signal processing. Repetitive acoustic stimulation, cortical electrical stimulation or auditory fear conditioning evokes plastic changes of the central auditory system. The changes are based upon egocentric selection evoked by focused positive feedback associated with lateral inhibition. Focal electric stimulation of the auditory cortex evokes short-term changes in the auditory 1p MON. PM cortex and subcortical auditory nuclei. An increase in a cortical acetylcholine level during the electric stimulation changes the cortical changes from short-term to long-term. There are two types of plastic changes ͑reorganizations͒: centripetal best frequency shifts for expanded reorganization of a neural frequency map and centrifugal best frequency shifts for compressed reorganization of the map. -

Krogh's Principle
Introduction to Neuroscience: Behavioral Neuroscience Neuroethology, Comparative Neuroscience, Natural Neuroscience Nachum Ulanovsky Department of Neurobiology, Weizmann Institute of Science 2017-2018, 2nd semester Principles of Neuroethology Neuroethology seeks to understand the mechanisms by which the Neurobiology central nervous system controls the Neuroethology Ethology natural behavior of animals. • Focus on Natural behaviors: Choosing to study a well-defined and reproducible yet natural behavior (either Innate or Learned behavior) • Need to study thoroughly the animal’s behavior, including in the field: Neuroethology starts with a good understanding of Ethology. • If you study the animals in the lab, you need to keep them in conditions as natural as possible, to avoid the occurrence of unnatural behaviors. • Krogh’s principle 1 Krogh’s principle August Krogh Nobel prize 1920 “For such a large number of problems there will be some animal of choice or a few such animals on which it can be most conveniently studied. Many years ago when my teacher, Christian Bohr, was interested in the respiratory mechanism of the lung and devised the method of studying the exchange through each lung separately, he found that a certain kind of tortoise possessed a trachea dividing into the main bronchi high up in the neck, and we used to say as a laboratory joke that this animal had been created expressly for the purposes of respiration physiology. I have no doubt that there is quite a number of animals which are similarly "created" for special physiological -

Lateral Inhibition Effects Demonstrate That the Maturational State of Neurons That Then Inhibit Their Neighbors
522 Lateral Inhibition effects demonstrate that the maturational state of neurons that then inhibit their neighbors. When a the learner’s brain is crucial for the attainment of stimulus (such as a bar of light or any other stim- a language system. ulus) excites a number of neurons in the network Several questions remain open about the mecha- (in this case neurons 4e, 5e, 6e, and 7e), the effect nisms underlying language learning. One issue is of inhibition is to suppress the neurons just out- whether specific aspects of language acquisition side the edge of the bar (3e and 8e) because those should be attributed to language-specific versus neurons are inhibited but not excited. Further, general-purpose learning mechanisms. Another issue because the neurons just inside the edges of the is whether children’s native language can affect the bar (4e and 7e) are excited by light and only inhib- way they think, and whether language is necessary ited by one neighbor, they are especially active. or helpful for the development of human concepts. This leads to perceptual contrast enhancement at borders. Further research showed that lateral inhi- Anna Papafragou bition also applied to overlapping stimuli, and that its strength fell off with distance between the See also Aphasias; Audition: Cognitive Influences; Context Effects in Perception; Speech Perception; Top- interacting stimuli. Down and Bottom-Up Processing; Word Recognition Haldan Keffer Hartline won the Nobel Prize in 1967 for discovering lateral inhibition and its neu- ral correlates. The first inhibitory circuit in the Further Readings nervous system was found in the horseshoe crab (Limulus polyphemus). -

Sensory Receptors A17 (1)
SENSORY RECEPTORS A17 (1) Sensory Receptors Last updated: April 20, 2019 Sensory receptors - transducers that convert various forms of energy in environment into action potentials in neurons. sensory receptors may be: a) neurons (distal tip of peripheral axon of sensory neuron) – e.g. in skin receptors. b) specialized cells (that release neurotransmitter and generate action potentials in neurons) – e.g. in complex sense organs (vision, hearing, equilibrium, taste). sensory receptor is often associated with nonneural cells that surround it, forming SENSE ORGAN. to stimulate receptor, stimulus must first pass through intervening tissues (stimulus accession). each receptor is adapted to respond to one particular form of energy at much lower threshold than other receptors respond to this form of energy. adequate (s. appropriate) stimulus - form of energy to which receptor is most sensitive; receptors also can respond to other energy forms, but at much higher thresholds (e.g. adequate stimulus for eye is light; eyeball rubbing will stimulate rods and cones to produce light sensation, but threshold is much higher than in skin pressure receptors). when information about stimulus reaches CNS, it produces: a) reflex response b) conscious sensation c) behavior alteration SENSORY MODALITIES Sensory Modality Receptor Sense Organ CONSCIOUS SENSATIONS Vision Rods & cones Eye Hearing Hair cells Ear (organ of Corti) Smell Olfactory neurons Olfactory mucous membrane Taste Taste receptor cells Taste bud Rotational acceleration Hair cells Ear (semicircular -

Building Machines That Learn and Think Like People
BEHAVIORAL AND BRAIN SCIENCES (2017), Page 1 of 72 doi:10.1017/S0140525X16001837, e253 Building machines that learn and think like people Brenden M. Lake Department of Psychology and Center for Data Science, New York University, New York, NY 10011 [email protected] http://cims.nyu.edu/~brenden/ Tomer D. Ullman Department of Brain and Cognitive Sciences and The Center for Brains, Minds and Machines, Massachusetts Institute of Technology, Cambridge, MA 02139 [email protected] http://www.mit.edu/~tomeru/ Joshua B. Tenenbaum Department of Brain and Cognitive Sciences and The Center for Brains, Minds and Machines, Massachusetts Institute of Technology, Cambridge, MA 02139 [email protected] http://web.mit.edu/cocosci/josh.html Samuel J. Gershman Department of Psychology and Center for Brain Science, Harvard University, Cambridge, MA 02138, and The Center for Brains, Minds and Machines, Massachusetts Institute of Technology, Cambridge, MA 02139 [email protected] http://gershmanlab.webfactional.com/index.html Abstract: Recent progress in artificial intelligence has renewed interest in building systems that learn and think like people. Many advances have come from using deep neural networks trained end-to-end in tasks such as object recognition, video games, and board games, achieving performance that equals or even beats that of humans in some respects. Despite their biological inspiration and performance achievements, these systems differ from human intelligence in crucial ways. We review progress in cognitive science suggesting that truly human-like -

The Retina the Retina the Pigment Layer Contains Melanin That Prevents Light Reflection Throughout the Globe of the Eye
The Retina The retina The pigment layer contains melanin that prevents light reflection throughout the globe of the eye. It is also a store of Vitamin A. Rods and Cones Photoreceptors of the nervous system responsible for transforming light energy into the electrical energy of the nervous system. Bipolar cells Link between photoreceptors and ganglion cells. Horizontal cells Provide inhibition between bipolar cells. This is the mechanism of lateral inhibition which is important to edge detection and contrast enhancement. Amacrine cells Transmit excitatory signals from bipolar to ganglion cells. Thought to be important in signalling changes in light intensity. Neural circuitry About 125 rods and 5 cones converge on each optic nerve fibre. In the central portion of the fovea there are no rods. The ratio of cones to optic nerves in the fovea is one. This increases the visual acuity of the fovea. Rods and Cones Photosensitive substance in rods is called rhodopsin and in cones is called iodopsin. The Photochemistry of Vision Rhodopsin and Iodopsin Cis-retinal +Opsin Light sensitive Light Trans-retinal Opsin Light insensitive This is a reversible reaction Cis-retinal +Opsin Light sensitive Vitamin A In the presence of light, the rods and cones are hyperpolarized mv t Hyperpolarizing potential lasts for upto half a second, leading to the perception of fusion of flickering lights. Receptor potential – rod and cone potential Hyperpolarizing receptor potential caused by rhodopsin decomposition. Hyperpolarizing potential lasts for upto half a second, leading to the fusion of flickering lights. Light and dark adaptation in the visual system The eye is capable of vision in conditions of light that vary greatly in intensity The eye adapts dynamically to lighting conditions Light and Dark Adaptation We are able to see in light intensities that vary greatly. -

Small and Dim Target Detection Via Lateral Inhibition Filtering and Artificial Bee Colony Based Selective Visual Attention
Small and Dim Target Detection via Lateral Inhibition Filtering and Artificial Bee Colony Based Selective Visual Attention Haibin Duan1,2*, Yimin Deng1,2, Xiaohua Wang2, Chunfang Xu1 1 State Key Laboratory of Virtual Reality Technology and Systems, School of Automation Science and Electrical Engineering, Beihang University (formerly Beijing University of Aeronautics and Astronautics), Beijing, P. R. China, 2 Science and Technology on Aircraft Control Laboratory, Beihang University, Beijing, P. R. China Abstract This paper proposed a novel bionic selective visual attention mechanism to quickly select regions that contain salient objects to reduce calculations. Firstly, lateral inhibition filtering, inspired by the limulus’ ommateum, is applied to filter low- frequency noises. After the filtering operation, we use Artificial Bee Colony (ABC) algorithm based selective visual attention mechanism to obtain the interested object to carry through the following recognition operation. In order to eliminate the camera motion influence, this paper adopted ABC algorithm, a new optimization method inspired by swarm intelligence, to calculate the motion salience map to integrate with conventional visual attention. To prove the feasibility and effectiveness of our method, several experiments were conducted. First the filtering results of lateral inhibition filter were shown to illustrate its noise reducing effect, then we applied the ABC algorithm to obtain the motion features of the image sequence. The ABC algorithm is proved to be more robust and effective through the comparison between ABC algorithm and popular Particle Swarm Optimization (PSO) algorithm. Except for the above results, we also compared the classic visual attention mechanism and our ABC algorithm based visual attention mechanism, and the experimental results of which further verified the effectiveness of our method. -

Ingenieurfakultät Bau Geo Umwelt
Technische Universität München Ingenieurfakultät Bau Geo Umwelt Lehrstuhl für Wasserbau und Wasserwirtschaft Rolling stones Modelling sediment in gravel bed rivers Markus Andreas Reisenbüchler Vollständiger Abdruck der von der Ingenieurfakultät Bau Geo Umwelt der Technischen Universität München zur Erlangung des akademischen Grades eines Doktor-Ingenieurs genehmigten Dissertation. Vorsitzender: Prof. Dr. Kurosch Thuro Prüfer der Dissertation: 1. Prof. Dr.sc.tech. Peter Rutschmann 2. Prof. Dr. Helmut Habersack 3. Prof. Dr.-Ing. Dr.h.c. mult. Franz Nestmann Die Dissertation wurde am 10.06.2020 bei der Technischen Universität München ein- gereicht und durch die Ingenieurfakultät Bau Geo Umwelt am 08.10.2020 angenommen. Dies ist eine kumulative Dissertation basierend auf Veröffentlichungen in interna- tionalen Fachzeitschriften. III Acknowledgement First and foremost, I would like to thank my Mentor Dr.-Ing. Minh Duc Bui and Supervisor Prof. Dr. Peter Rutschmann for the offer and opportunity to do a dissertation. During the study, they supported me with guidance, mentoring, and encouragement. Special gratitude goes to Dr.-Ing. Daniel Skublics, whose expertise on the study site helped me a lot during this work. I am also grateful to my colleagues and staff members at the Chair of Hydraulic and Water Resources Engineering, for their help in my research but also for the common activities besides the work. Finally, I’m deeply grateful to my family and my girlfriend Isabella for supporting me during this intensive and valuable time. Markus Andreas Reisenbüchler Email: [email protected] V Abstract The precise description of our environment is highly important, as our civilisation is very sensitive to changes in natural systems. -

Feed-Forward, Feed-Back, and Distributed Feature Representation During Visual Word Recognition Revealed by Human Intracranial Neurophysiology
Feed-forward, feed-back, and distributed feature representation during visual word recognition revealed by human intracranial neurophysiology Laura Long Columbia University Minda Yang Columbia University Nikolaus Kriegeskorte Columbia University https://orcid.org/0000-0001-7433-9005 Joshua Jacobs Columbia University https://orcid.org/0000-0003-1807-6882 Robert Remez Barnard College, Columbia University Michael Sperling Thomas Jefferson University https://orcid.org/0000-0003-0708-6006 Ashwini Sharan Jefferson Medical College Bradley Lega UT Southwestern Medical Center Alexis Burks University of Texas Southwestern Medical Center Gregory Worrell Mayo Clinic Robert Gross Emory University Barbara Jobst Geisel School of Medicine at Dartmouth Kathryn Davis University of Pennsylvania Kareem Zaghloul National Institutes of Health https://orcid.org/0000-0001-8575-3578 Sameer Sheth Department of Neurosurgery, Baylor College of Medicine Joel Stein Page 1/18 Hospital of the University of Pennsylvania Sandhitsu Das Hospital of the University of Pennsylvania Richard Gorniak Thomas Jefferson University Hospital Paul Wanda University of Pennsylvania Michael Kahana University of Pennsylvania Nima Mesgarani ( [email protected] ) Columbia University Article Keywords: visual word recognition, human intracranial neurophysiology, feed-forward processing, feedback processing, anatomically distributed processing DOI: https://doi.org/10.21203/rs.3.rs-95141/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 2/18 Abstract Scientists debate where, when, and how different visual, orthographic, lexical, and semantic features are involved in visual word recognition. In this study, we investigate intracranial neurophysiology data from 151 patients engaged in reading single words. Using representational similarity analysis, we characterize the neural representation of a hierarchy of word features across the entire cerebral cortex.