<Abstract Centered> an ABSTRACT of the THESIS OF
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

BJCP Mead Exam Study Guide
BJCP Mead Exam Study Guide What you need to know to pass the Mead Exam September 2009 Initial release: September 22, 2009 Contributing Authors Gordon Strong Susan Ruud Kristen England Ken Schramm Curt Stock Petar Bakulić Michael Zapolski, Sr. (Hightest) Copyright © 2009 by the authors and the BJCP TABLE OF CONTENTS 1. Introduction............................................................................................................................. 1 1.1. Recommended Reading.................................................................................................. 1 1.2. Advanced Reading.......................................................................................................... 3 1.3. Important BJCP References............................................................................................ 3 2. The BJCP Mead Exam............................................................................................................ 4 2.1. Mead Exam Details......................................................................................................... 4 2.1.1. Written Section..................................................................................................... 4 2.1.2. Tasting Section..................................................................................................... 5 2.2. BJCP Mead Judge Question Pool ................................................................................... 5 2.2.1. Section 1.............................................................................................................. -
Taste and Smell Laboratory Experiments Activity 1
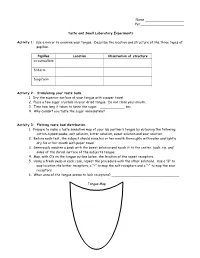
Name ___________________ Per._____________________ Taste and Smell Laboratory Experiments Activity 1 : Use a mirror to examine your tongue. Describe the location and structure of the three types of papillae. Papillae Location Observation of structure circumvallate filiform fungiform Activity 2: Stimulating your taste buds. 1. Dry the superior surface of your tongue with a paper towel. 2. Place a few sugar crystals on your dried tongue. Do not close your mouth. 3. Time how long it takes to taste the sugar. ____________ sec. 4. Why couldn’t you taste the sugar immediately? Activity 3: Plotting taste bud distribution. 1. Prepare to make a taste sensation map of your lab partner’s tongue by obtaining the following: cotton-tipped swabs, salt solution, bitter solution, sweet solution and sour solution. 2. Before each test, the subject should rinse his or her mouth thoroughly with water and lightly dry his or her mouth with paper towel. 3. Generously moisten a swab with the sweet solution and touch it to the center, back, tip, and sides of the dorsal surface of the subject’s tongue. 4. Map, with O’s on the tongue outline below, the location of the sweet receptors. 5. Using a fresh swab in each case, repeat the procedure with the other solutions. Use a “B” to map location the bitter receptors, a “+” to map the salt receptors and a “–“ to map the sour receptors. 6. What area of the tongue seems to lack receptors? _________________________________ Tongue Map Activity 4: Examining the combined effects of smell and texture on taste. 1. Ask the subject to sit with eyes closed and to pinch his or her nostrils shut. -

Loss of Taste Is Very Common After HCT: Taste in Wine Is Very Important
Bone Marrow Transplantation (2020) 55:995–996 https://doi.org/10.1038/s41409-019-0775-y EDITORIAL Loss of taste is very common after HCT: taste in wine is very important. Shaun R. McCann1 Received: 24 November 2019 / Revised: 26 November 2019 / Accepted: 3 December 2019 / Published online: 17 December 2019 © Springer Nature Limited 2019 ‘The first taste of a wine is like the first kiss; you look Of course, taste and smell are intimately related [4] forward to the second’. and smelling a wine cork or a small sample of wine, Andre Tcelistcheff. American winemaker. 1901–1994 without swilling, to detect contamination with TCA (2,4,6—Trichloroanisole) is paramount (Fig. 1). We are all Tchelistcheff’s words remind me of the lyrics ‘… a kiss is familiar with the tongue map wherein certain areas of the just a kiss…’ from the song ‘As Time Goes by’ written by tongue were believed to detect certain tastes, (sweetness, Herman Hupfeld in 1931 and made famous in the film sourness, bitterness and saltiness), with bitterness at the 1234567890();,: 1234567890();,: ‘Casablanca’ in 1942 sung by Sam (played by the American back, sourness and saltiness at the sides and sweetness at actor, singer and musician Arthur ‘Dooley’ Wilson) in the tip (Fig. 2). You can add ‘umami’ to this list a word Rick’s Café. derived from Japanese that refers to detection of a savoury Most physicians who look after patients who have taste (yes, I only learnt this word when researching this received a haematopoietic cell transplant (HCT) are not particularly concerned with dysgeusia (a distortion of the sense of taste). -

Oral Sensation
Oral sensation Beata Keremi DMD, PhD Department of Oral Biology General sensation in the oral cavity Protopathic: – touch A beta and A delta fibers – pressure (II and III types sensory neurons) – temperature – pain Epicritic: – stereognosia: detect the shape of objects General sensation in the oral cavity http://joecicinelli.com/homunculus-training/ Temperature sensation mucous membranes – very sensitive protective mechanism receptors are bare nerve endings – cold receptor - below a threshold – hot receptor - above a threshold – skin temperature receptor - around 25ºC - same on the lips – maximum tolerable temperature: 70-80 ºC A delta and C fibers – relatively slow (III or IV types sensory neurons) Pressure sensation teeth are sensitive – axially and laterally applied pressure – receptors in the periodontal ligaments end-organs of the Ruffini type - A beta nerve fibers – slow adaptation http://faculty.washington.edu/chudler/receptor.html http://people.usd.edu/~cliff/Courses/Behavioral%20Neuroscience/Lordosis/Lordosisfigs/lorsenspics.html excitability depend on the position Proprioception Proprioceptive impulses from: – muscles of the tongue Muscle spindle – masticatory muscles • elevator • depressor - few – temporomandibular joint http://medical-dictionary.thefreedictionary.com/muscle+spindle Neuropeptide - suggesting the presence of nerve endings – joint capsules – synovial discs Taste and smell Nose and mouth are sentinels of chemical world In dysfunction can be an early indicator of serious diseases – Alzheimer’s; Parkinson’s diseases -

Molecular Gastronomy and Sensory Science
Sensory Science – myth busting Jannie Vestergaard Michael Bom Frøst, PhD Project manager Associate Professor, Director of Studies Taste Skåne Department of Food Science Skåne food Innovation Sensory Science Group Network [email protected] jannie.vestergaard@ livsmedelsakademin.se OVERVIEW • An envelope with unusual content • The senses at work in real life • Matching coffee with foods • Complexity enters the equation – Is really good coffee complex? • Learning to taste – train and be challenged – Tricks of the sensory trade The envelope Taste kit for the lecture 1. Take a piece of chewing gum (the white rectangular piece) Nordic Barista Cup, August 25 2011, Jannie Vestergaard & Michael Bom Frøst 3 THE SENSES AT WORK IN REAL LIFE Three basic properties by sense perception The quality (Qualitas) • Identification • What is the property? • Recognition of objects (= a number of properties) Intensity (magnitude = the size) • How much is there of it? Affective dimension • Affect: The experience of emotion • Is it something I like/don’t like Nordic Barista Cup, August 25 2011, Jannie Vestergaard & Michael Bom Frøst 5 The senses – 5 classic + some less known The remote senses Vision The higher senses Audition Olfaction The lower The near senses senses Gustation The chemical Somesthesis ( ~ sense of touch) senses Pressure Temperature Pain / irritation / chemesthesis Balance Body and joint position Nordic Barista Cup, August 25 2011, Jannie Vestergaard & Michael Bom Frøst 6 Taste – Myth busting : The tongue map The different basic tastes are experienced at different locations in the mouth: Bitter only in the back Tongues on scientific evidence: Take out the chewing gum Apply the filter paper to the tip of your tongue Taste-receptor cells, buds and papillae. -

Sensation and Perception
Sensation and Perception OUTLINE OF RESOURCES Introducing Sensation and Perception Podcast/Lecture/Discussion Topic: Person Perception (p. 3) UPDATED Basic Principles of Sensation and Perception Lecture/Discussion Topics: Sensation Versus Perception (p. 4) Top-Down Processing (p. 5) “Thin-Slicing” (p. 6) Classroom Exercises: A Scale to Assess Sensory-Processing Sensitivity (p. 6) UPDATED Human Senses Demonstration Kits (p. 6) UPDATED Classroom Exercises/Student Projects: The Wundt-Jastrow Illusion (p. 4) LaunchPad Video: The Man Who Cannot Recognize Faces* Thresholds Lecture/Discussion Topics: Gustav Fechner and Psychophysics (p. 7) Subliminal Smells (p. 8) Subliminal Persuasion (p. 9) Applying Weber’s Law (p. 10) Student Projects: The Variability of the Absolute Threshold (p. 8) Understanding Weber’s Law (p. 9) Sensory Adaptation Student Project: Sensory Adaptation (p. 10) UPDATED Classroom Exercises: Eye Movements (p. 10) Sensory Adaptation in the Marketplace (p. 11) Perceptual Set Lecture/Discussion Topic: Do Red Objects Feel Warmer or Colder Than Blue Objects? (p. 11) NEW Classroom Exercises: Perceptual Set (p. 11) UPDATED Perceptual Set and Gender Stereotypes (p. 12) Context Effects (see also Brightness Contrast, p. 22) Lecture/Discussion Topic: Context and Perception (p. 13) Vision: Sensory and Perceptual Processing Classroom Exercise/Student Project: Physiology of the Eye—A CD-ROM for Teaching Sensation and Perception (p. 13) LaunchPad Video: Vision: How We See* The Eye and the Stimulus Input Lecture/Discussion Topic:Classroom as Eyeball (p. 13) Student Projects: Color the Eyeball (p. 13) NEW Locating the Retinal Blood Vessels (p. 13) Student Projects/Classroom Exercises: Rods, Cones, and Color Vision (p. 14) UPDATED Locating the Blind Spot (p. -

The Receptors and Cells for Mammalian Taste Jayaram Chandrashekar1, Mark A
INSIGHT REVIEW NATURE|Vol 444|16 November 2006|doi:10.1038/nature05401 The receptors and cells for mammalian taste Jayaram Chandrashekar1, Mark A. Hoon2, Nicholas J. P. Ryba2 & Charles S. Zuker1 The emerging picture of taste coding at the periphery is one of elegant simplicity. Contrary to what was generally believed, it is now clear that distinct cell types expressing unique receptors are tuned to detect each of the five basic tastes: sweet, sour, bitter, salty and umami. Importantly, receptor cells for each taste quality function as dedicated sensors wired to elicit stereotypic responses. Our sensory systems are responsible for generating an internal represen- The anatomical substrates and units of taste detection are taste-recep- tation of the outside world, including its chemical (taste and olfaction) tor cells (TRCs; Fig. 1). TRCs are assembled into taste buds, which are and physical (mechanical, sound, vision and temperature) features. In distributed across different papillae of the tongue and palate epithelium. this review we examine recent advances in our understanding of the How are the different tastes detected, and how is taste quality represented? biology of taste, focusing on receptors, cells and the logic of taste cod- In the simplest scenario, sweet, bitter, sour, salty and umami tastants ing at the periphery. would each be recognized by different cells expressing specialized recep- Taste is in charge of evaluating the nutritious content of food and pre- tors. Coding at the periphery could then rely on straightforward labelled venting the ingestion of toxic substances. Sweet taste permits the identi- lines (that is, independent sweet, bitter, sour, salty and umami signals) fication of energy-rich nutrients, umami allows the recognition of amino to transform tastant quality into neural signals (Fig. -

Physical Approaches to Masking Bitter Taste: Lessons from Food and Pharmaceuticals
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Pharm Res Manuscript Author . Author manuscript; Manuscript Author available in PMC 2016 June 08. Published in final edited form as: Pharm Res. 2014 November ; 31(11): 2921–2939. doi:10.1007/s11095-014-1480-6. Physical Approaches to Masking Bitter Taste: Lessons from Food and Pharmaceuticals John N. Coupland and John E. Hayes Department of Food Science, The Pennsylvania State University 337 Food Science Building, University Park, Pennsylvania 16802, USA John N. Coupland: [email protected] Abstract Many drugs and desirable phytochemicals are bitter, and bitter tastes are aversive. Food and pharmaceutical manufacturers share a common need for bitterness-masking strategies that allow them to deliver useful quantities of the active compounds in an acceptable form and in this review we compare and contrast the challenges and approaches by researchers in both fields. We focus on physical approaches, i.e., micro- or nano-structures to bind bitter compounds in the mouth, yet break down to allow release after they are swallowed. In all of these methods, the assumption is the degree of bitterness suppression depends on the concentration of bitterant in the saliva and hence the proportion that is bound. Surprisingly, this hypothesis has only rarely been fully tested using a combination of adequate human sensory trials and measurements of binding. This is especially true in pharmaceutical systems, perhaps due to the greater experimental challenges in sensory analysis of drugs. Keywords bitter; emulsion; encapsulation; food; taste INTRODUCTION The development of bitter taste can be understood from the perspective of the eater or the eaten. -

Gustatory Dysfunction
Dental Science - Review Article Gustatory dysfunction T. Maheswaran, P. Abikshyeet1, G. Sitra2, S. Gokulanathan3, V. Vaithiyanadane4, S. Jeelani2 Department of ABSTRACT Oral Pathology and Tastes in humans provide a vital tool for screening soluble chemicals for food evaluation, selection, and avoidance Microbiology, Vivekanandha Dental College for of potentially toxic substances. Taste or gustatory dysfunctions are implicated in loss of appetite, unintended Women, Elayampalayam, weight loss, malnutrition, and reduced quality of life. Dental practitioners are often the first clinicians to be Tiruchengode, 1Kalinga presented with complaints about taste dysfunction. This brief review provides a summary of the common causes Institute of Dental Sciences, of taste disorders, problems associated with assessing taste function in a clinical setting and management Bhubaneswar, 2Oral options available to the dental practitioner. Medicine and Radiology, Indira Gandhi Institute of Dental Sciences, Puducherry, 3Periodontics, Vivekanandha Dental College for Women, Elayampalayam, Tiruchengode, 4Physiology, Vivekanandha Dental College for Women, Elayampalayam, Tiruchengode, India Address for correspondence: Dr. T. Maheswaran, E-mail: maheswaranmds@ yahoo.in Received : 30-03-14 Review completed : 30-03-14 Accepted : 09-04-14 KEY WORDS: Ageusia, dysgeusia, hypergeusia, hypogeusia, phantogeusia, taste disorders, taste disturbances he chemosensory functions of taste and smell play a vital report increased use of sugar and salt to compensate for T role in -

Five Flavors: How the Palate Changes
Five Flavors: How the Palate Changes TASTE Putting together a meal is more than making the plate look pretty! Taste is connected to memory retrieval. Researchers have found that the experience also lies in satisfying all the senses. We may also hear the food we prepare. Our senses open as we listen to cabinets and refrigerator doors open. We hear pots and pans and dishes used for preparing for a meal. We hear cutting and washing of vegetables and other foods. And then comes the smell and taste. Smelling sweet, savory, and spicy alerts the brain that we are preparing to eat something delicious. Beyond sight, tasting brings all the senses together. With tast- ing, we complete the expectation and taste sweet, sour, salty, and bitter. SIGHT Dine-in-the-dark restaurant: Where the food tastes disappointing, but why? • The color of food can change our perception; e.g. if something is green, it is fresh and has better nutrition • People perceive that hot chocolate tastes better out of a cream or orange colored cup compared to a different color. • Research indicates sugar solutions with a reddish hue tastes sweeter like a fruit punch. University of Illinois-Champaign College of Agriculture, Consumer and Environmental Sciences. United States Department of Agriculture/Local Extension Councils Cooperating. University of Illinois Extension provides equal opportunities in programs and employment MOUTH WATERING Like taste, our sense of smell is also closely linked to our emotions. This is because both senses are connect- ed to the involuntary nervous system. That is why a bad taste or odor can bring about vomiting or nausea. -

Taste Perception with Age Promotores: Prof
Taste perception with age Promotores: Prof. dr. J.H.A. Kroeze Hoogleraar Psychologische en Sensorische Aspecten van Voeding, Wageningen Universiteit Prof. dr. A.G.J. Voragen Hoogleraar Levensmiddelenchemie Wageningen Universiteit Leden Promotiecommissie : Prof. dr. C. Murphy San Diego State University, USA Prof. dr. L.J. Frewer Wageningen Universiteit Dr. ir. C. de Graaf Wageningen Universiteit Prof. dr. E.P. Köster Universiteit Utrecht Taste perception with age Jozina Mojet Proefschrift Ter verkrijging van de graad van doctor op gezag van de rector magnificus van Wageningen Universiteit, prof. dr. ir. L. Speelman, In het openbaar te verdedigen op vrijdag 7 mei 2004 des namiddags te vier uur in de Aula ISBN: 90-8504-058-2 Mojet, J. (2004) Taste perception with age PhD thesis, Wageningen University, The Netherlands Keywords: age, thresholds, supra-threshold intensities, pleasantness, optimally preferred concentration, olfactory deprivation, signal-to-noise ratio This thesis describes experiments studying age-related differences in taste perception. Thresholds, supra-threshold intensities and pleasantness for ten different tastants, representing the five taste qualities sweet, sour, salty, bitter and umami were measured in the same group of elderly and young subjects. The tastants were dissolved in water and in product. Age has a deteriorating effect on taste sensitivity, which is already noticeable in the young-old group of elderly. First, this effect is generic in nature, i.e. more than 90% of the total variance attributable to age is due to age alone and less than 6% to age-related differences in the perception of the different taste qualities. Secondly, the differentiation between the different taste qualities is less distinct for elderly than for young people. -

Sensation and Perception
MYTH SCIENCE? Is it true . That subliminal messages in advertising can make you buy particular products? That an object’s color is not an intrinsic property of the object? That pheromones can make some people irresistible to members of the preferred sex? That different tastes are detected on different parts of your tongue? That most psychologists study ESP? That magnets can relieve pain? (inset) monkeybusinessimages/Getty Images Images (bkgrd) Erik Isakson/Tetraimages/Getty Erik Isakson/Tetra images/Getty Images Sensation and Perception adult, he earned a master’s degree in It was through skiing that Mike met international affairs from Johns Hop- his wife, Jennifer. An accomplished kins University, went to work for the skier herself, she volunteered to be Learning to See CIA, and then became a successful his guide at a ski slope. Today, Mike Mike was just three years old when businessman. and Jennifer and their two sons are all a jar of chemicals, left in an old He also learned to skydive, wind- avid skiers ( Abrams, 2002 ). storage shed, exploded in his face. surf, water ski, and snow ski. How does In his forties, Mike started a suc- The blast destroyed his left eye and a blind person ski down mountains? If cessful company that develops global severely damaged his right eye. For you answered, “Very carefully,” you’d positioning devices— along with more than four decades, Mike May be wrong— at least in Mike’s case. With other mobility devices— for the blind. was completely blind ( Kurson, 2007 ). a guide skiing in front of him shouting The portable navigation system gives But despite his blindness, Mike expe- “left” or “right” to identify obstacles, visually impaired people informa- rienced—and accomplished—much Mike hurtled down the most difficult tion about their location, landmarks, more than most people ever dream black diamond slopes at speeds up streets, and so forth wherever they of achieving.