Plant Derived Alkaloids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Title ALKALOID BIOSYNTHESIS in CULTURED TISSUES OF
ALKALOID BIOSYNTHESIS IN CULTURED TISSUES OF Title DUBOISIA( Dissertation_全文 ) Author(s) Endo, Tsuyoshi Citation 京都大学 Issue Date 1989-03-23 URL https://doi.org/10.14989/doctor.k4307 Right Type Thesis or Dissertation Textversion author Kyoto University ALKALOID BIOSYNTHESIS IN C;ULTURED TISSUES OF DUBOISIA . , . ; . , " 1. :'. '. o , " ::,,~./ ~ ~';-~::::> ,/ . , , .~ - '.'~ . / -.-.........."~l . ~·_l:""· .... : .. { ." , :: I i i , (, ' ALKALOID BIOSYNTHESIS IN CULTURED TISSUES OF DUBOISIA TSUYOSHIENDO 1989 CONTENTS INTRODUCTION ----------1 CHAPTER I ALKALOID PRODUCTION IN CULTURED DUBOISIA TISSUES. INTRODUCTION ----------6 SECTION 1 Alkaloid Production and Plant Regeneration from ~ leichhardtii Calluses. ----------8 SECTION 2 Alkaloid Production in Cultured Roots of Three Species of Duboisia. ---------16 SECTION 3 Non-enzymatic Synthesis of Hygrine from Acetoacetic Acid and from Acetonedicar- boxylic Acid. ---------25 CHAPTER II SOMATIC HYBRIDIZATION OF DUBOISIA AND NICOTIANA. INTRODUCTION ---------35 SECTION 1 Establishment of an Intergeneric Hybrid Cell Line of ~ hopwoodii and ~ tabacum. ---------38 SECTION 2 Genetic Diversity Originating from a Single Somatic Hybrid Cell. ---------47 SECTION 3 Alkaloid Biosynthesis in Somatic Hybrids, D. leichhardtii + ~ tabacum ---------59 CONCLUSIONS ---------76 ACKNOWLEDGMENTS ---------79 REFERENCES ---------80 PUBLICATIONS ---------90 ABBREVIATIONS BA 6-benzyladenine OAPI 4',6-diamino-2-phenylindoledihydrochloride EDTA ethylenediaminetetraacetic acid GC-MS gas chromatography - mass spectrometry -

Summary of Offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019
Summary of offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019 3841 Number of items in BX 301 thru BX 463 1815 Number of unique text strings used as taxa 990 Taxa offered as bulbs 1056 Taxa offered as seeds 308 Number of genera This does not include the SXs. Top 20 Most Oft Listed: BULBS Times listed SEEDS Times listed Oxalis obtusa 53 Zephyranthes primulina 20 Oxalis flava 36 Rhodophiala bifida 14 Oxalis hirta 25 Habranthus tubispathus 13 Oxalis bowiei 22 Moraea villosa 13 Ferraria crispa 20 Veltheimia bracteata 13 Oxalis sp. 20 Clivia miniata 12 Oxalis purpurea 18 Zephyranthes drummondii 12 Lachenalia mutabilis 17 Zephyranthes reginae 11 Moraea sp. 17 Amaryllis belladonna 10 Amaryllis belladonna 14 Calochortus venustus 10 Oxalis luteola 14 Zephyranthes fosteri 10 Albuca sp. 13 Calochortus luteus 9 Moraea villosa 13 Crinum bulbispermum 9 Oxalis caprina 13 Habranthus robustus 9 Oxalis imbricata 12 Haemanthus albiflos 9 Oxalis namaquana 12 Nerine bowdenii 9 Oxalis engleriana 11 Cyclamen graecum 8 Oxalis melanosticta 'Ken Aslet'11 Fritillaria affinis 8 Moraea ciliata 10 Habranthus brachyandrus 8 Oxalis commutata 10 Zephyranthes 'Pink Beauty' 8 Summary of offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019 Most taxa specify to species level. 34 taxa were listed as Genus sp. for bulbs 23 taxa were listed as Genus sp. for seeds 141 taxa were listed with quoted 'Variety' Top 20 Most often listed Genera BULBS SEEDS Genus N items BXs Genus N items BXs Oxalis 450 64 Zephyranthes 202 35 Lachenalia 125 47 Calochortus 94 15 Moraea 99 31 Moraea -

Duboisia Myoporoides R.Br. Family: Solanaceae Brown, R
Australian Tropical Rainforest Plants - Online edition Duboisia myoporoides R.Br. Family: Solanaceae Brown, R. (1810) Prodromus Florae Novae Hollandiae : 448. Type: New South Wales, Port Jackson, R. Brown, syn: BM, K, MEL, NSW, P. (Fide Purdie et al. 1982.). Common name: Soft Corkwood; Mgmeo; Poison Corkwood; Poisonous Corkwood; Corkwood Tree; Eye-opening Tree; Eye-plant; Duboisia; Yellow Basswood; Elm; Corkwood Stem Seldom exceeds 30 cm dbh. Bark pale brown, thick and corky, blaze usually darkening to greenish- brown on exposure. Leaves Leaf blades about 4-12 x 0.8-2.5 cm, soft and fleshy, indistinctly veined. Midrib raised on the upper surface. Flowers. © G. Sankowsky Flowers Small bell-shaped flowers present during most months of the year. Calyx about 1 mm long, lobes short, less than 0.5 mm long. Corolla induplicate-valvate in the bud. Induplicate sections of the corolla and inner surfaces of the corolla lobes clothed in somewhat matted, stellate hairs. Corolla tube about 4 mm long, lobes about 2 mm long. Fruit Fruits globular, about 6-8 mm diam. Seed and embryo curved like a banana or sausage. Seed +/- reniform, about 3-3.5 x 1 mm. Testa reticulate. Habit, leaves and flowers. © Seedlings CSIRO Cotyledons narrowly elliptic to almost linear, about 5-8 mm long. First pair of true leaves obovate, margins entire. At the tenth leaf stage: leaf blade +/- spathulate, apex rounded, base attenuate; midrib raised in a channel on the upper surface; petiole with a ridge down the middle. Seed germination time 31 to 264 days. Distribution and Ecology Occurs in CYP, NEQ, CEQ and southwards as far as south-eastern New South Wales. -

Annonaceae in the Western Pacific: Geographic Patterns and Four New
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: European Journal of Taxonomy Jahr/Year: 2017 Band/Volume: 0339 Autor(en)/Author(s): Turner Ian M., Utteridge M. A. Artikel/Article: Annonaceae in the Western Pacific: geographic patterns and four new species 1-44 © European Journal of Taxonomy; download unter http://www.europeanjournaloftaxonomy.eu; www.zobodat.at European Journal of Taxonomy 339: 1–44 ISSN 2118-9773 https://doi.org/10.5852/ejt.2017.339 www.europeanjournaloftaxonomy.eu 2017 · Turner I.M. & Utteridge T.M.A. This work is licensed under a Creative Commons Attribution 3.0 License. Research article Annonaceae in the Western Pacifi c: geographic patterns and four new species Ian M. TURNER 1,* & Timothy M.A. UTTERIDGE 2 1,2 Royal Botanic Gardens, Kew, Richmond, Surrey, TW9 3AE, UK. * Corresponding author: [email protected] 2 Email: [email protected] Abstract. The taxonomy and distribution of Pacifi c Annonaceae are reviewed in light of recent changes in generic delimitations. A new species of the genus Monoon from the Solomon Archipelago is described, Monoon salomonicum I.M.Turner & Utteridge sp. nov., together with an apparently related new species from New Guinea, Monoon pachypetalum I.M.Turner & Utteridge sp. nov. The confi rmed presence of the genus in the Solomon Islands extends the generic range eastward beyond New Guinea. Two new species of Huberantha are described, Huberantha asymmetrica I.M.Turner & Utteridge sp. nov. and Huberantha whistleri I.M.Turner & Utteridge sp. nov., from the Solomon Islands and Samoa respectively. New combinations are proposed: Drepananthus novoguineensis (Baker f.) I.M.Turner & Utteridge comb. -

ORNAMENTAL GARDEN PLANTS of the GUIANAS: an Historical Perspective of Selected Garden Plants from Guyana, Surinam and French Guiana
f ORNAMENTAL GARDEN PLANTS OF THE GUIANAS: An Historical Perspective of Selected Garden Plants from Guyana, Surinam and French Guiana Vf•-L - - •• -> 3H. .. h’ - — - ' - - V ' " " - 1« 7-. .. -JZ = IS^ X : TST~ .isf *“**2-rt * * , ' . / * 1 f f r m f l r l. Robert A. DeFilipps D e p a r t m e n t o f B o t a n y Smithsonian Institution, Washington, D.C. \ 1 9 9 2 ORNAMENTAL GARDEN PLANTS OF THE GUIANAS Table of Contents I. Map of the Guianas II. Introduction 1 III. Basic Bibliography 14 IV. Acknowledgements 17 V. Maps of Guyana, Surinam and French Guiana VI. Ornamental Garden Plants of the Guianas Gymnosperms 19 Dicotyledons 24 Monocotyledons 205 VII. Title Page, Maps and Plates Credits 319 VIII. Illustration Credits 321 IX. Common Names Index 345 X. Scientific Names Index 353 XI. Endpiece ORNAMENTAL GARDEN PLANTS OF THE GUIANAS Introduction I. Historical Setting of the Guianan Plant Heritage The Guianas are embedded high in the green shoulder of northern South America, an area once known as the "Wild Coast". They are the only non-Latin American countries in South America, and are situated just north of the Equator in a configuration with the Amazon River of Brazil to the south and the Orinoco River of Venezuela to the west. The three Guianas comprise, from west to east, the countries of Guyana (area: 83,000 square miles; capital: Georgetown), Surinam (area: 63, 037 square miles; capital: Paramaribo) and French Guiana (area: 34, 740 square miles; capital: Cayenne). Perhaps the earliest physical contact between Europeans and the present-day Guianas occurred in 1500 when the Spanish navigator Vincente Yanez Pinzon, after discovering the Amazon River, sailed northwest and entered the Oyapock River, which is now the eastern boundary of French Guiana. -

M.Sc. CHEMISTRY
M.Sc. CHEMISTRY ANALYTICAL CHEMISTRY SPECIALISATION SYLLABUS OF III & IV SEMESTERS REVISED AS PER NEW (CB) SYLLABUS FOR STUDENTS ADMITTED FROM THE YEAR 2016 ONWARDS M.Sc. CHEMISTRY (ANALYTICAL CHEMISTRY SPECIALISATION) Syllabus for III and IV Semesters (for the batches admitted in academic year 2016 & later under CBCS pattern) [Under Restructured CBCS Scheme] Grand total marks and credits (all 4 semesters) 2400 marks – 96 credits (Approved in the P.G. BOS meeting held on 01-07-2017) Semester - III (ANALYTICAL CHEMISTRY) [Under CBCS Scheme] (for the batches admitted in academic year 2016 & later under CBCS pattern) Hrs/week Internal assessment Semester exam Total Credits CH(AC)301T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)302T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)303T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)304T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)351P (LAB-I) 9 100 marks 4 CH(AC)352P (LAB-II) 9 100 marks 4 Total 600 marks 24 Semester - IV (ANALYTICAL CHEMISTRY) Hrs/week Internal assessment Semester exam Total Credits CH(AC)401T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)402T (core) 4 20 marks 80 marks 100 marks 4 CH(AC)403T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)404T (Elective) 4 20 marks 80 marks 100 marks 4 CH(AC)451P (LAB-I) 9 100 marks 4 CH(AC)452P (LAB-II) 9 100 marks 4 Total 600 marks 24 Grand total marks and credits (all 4 semesters) 2400 marks - 96 credits M.Sc.ANALYTICAL CHEMISTRY Semester III Paper I :CH (AC) 301T: CORE : Sampling, Data handling, Classical and Atomic spectral methods -

Original Article Effect of GABA on Blood Pressure and Blood Dynamics of Anesthetic Rats
Int J Clin Exp Med 2015;8(8):14296-14302 www.ijcem.com /ISSN:1940-5901/IJCEM0008622 Original Article Effect of GABA on blood pressure and blood dynamics of anesthetic rats Pengju Ma1, Ting Li2, Fanceng Ji3, Haibo Wang4, Juntao Pang4 1Department of Anesthesiogy, Anqiu People’s Hospital, Anqiu 262100, China; 2Delivery Room, People’s Hospital of Anqiu, Anqiu 262100, China; 3Department of Anesthesiogy, Weifang People’s Hospital, Weifang 261041, China; 4Department of Critical Care Medicine of Weifang People’s Hospital, Weifang 261041, China Received March 31, 2015; Accepted August 13, 2015; Epub August 15, 2015; Published August 30, 2015 Abstract: Background: This study aimed to investigate GABA effects on blood pressure and blood dynamics of an- esthetic rats by observing spontaneously hypertensive rats under both anesthesia and waking state. Materials and methods: 72 male waking Wistar-Kyokos (WKY) rats and 72 male anesthetized spontaneously hypertensive (SHR) rats were randomly divided into control group and experimental group (N = 36 each). Rats were further divided into three subgroups (N = 12 each), which received 15 μmol GABA, 35 nmol muscimol, or 4 nmol dicentrine into uni- lateral paraventricular nucleus, respectively. Rats in the control group (WKY1) and experimental group (SHR1) were compared for the GABA effect on blood pressure (MAP), heart rate (HR), and arterial baroreceptor reflex function (BRS) changes under waking state. Anesthetic WKY rats (WKY2) and spontaneously hypertensive rats (SHR2) were compared for the GABA effect on those abovementioned indexes. Abdominal aorta mean arterial pressure, heart rate, and arterial baroreceptor reflex function changes were compared in all rats. Results: MAP, HR, and BRS were slightly lower in the rats under anesthetic state than in waking state before treatment (P < 0.05); they did not show significant changes between anesthetic and waking state, however, after treatment (P > 0.05). -

Cocculus Laurifolius: a Rich Antimicrobial, Antioxidant and Phytochemical Source
Pak. J. Bot., 49(1): 337-344, 2017. COCCULUS LAURIFOLIUS: A RICH ANTIMICROBIAL, ANTIOXIDANT AND PHYTOCHEMICAL SOURCE MUHAMMAD AJAIB1*, ZUBARIA ASHRAF2 AND MUHAMMAD FAHEEM SIDDIQUI3 1Department of Botany (Bhimber Campus), Mirpur University of Science and Technology (MUST), Mirpur-10250 (AJK), Pakistan 2Department of Botany, GC University, Katchery Road, 54000, Lahore, Pakistan 3Department of Botany, University of Karachi, Karachi 75270, Pakistan *Corresponding author’s email: [email protected]; [email protected] Abstract The study was carried out to investigate the antimicrobial, antioxidant potential and the qualitative and quantitative phytochemical analysis of the bark and leaf of Cocculus laurifolius DC. by using polar and non-polar solvents, i.e. Petroleum ether, Chloroform, Methanol and distilled water. Chloroform bark extracts showed maximum % yield. Antimicrobial activity was determined by using 4 bacterial strains (2 gram-negative and 2 gram- positive) and 2 fungal strains. Leaf and bark extracts of C. laurifolius showed significant to average results against bacterial and fungal strain. Bark extracts of chloroform and methanol revealed a maximum zone of inhibition against S. aureus in agar-well diffusion method with values of 37±3.1mm and 37±2.2mm respectively and bark extract of methanol exhibited MIC value with 0.06±0.01 (at 0.9 mg/L) against E. coli. In antifungal activity, all extracts showed average results against fungal strains. Maximum result exhibited by bark extract of methanol with values 29±1.4 and 0.70±0.01 (at 1 mg/L) against F. solani in zone of inhibition and MIC analysis. Significant DPPH free radical scavenging activity of chloroform extracts of bark i.e. -

(12) United States Patent (10) Patent No.: US 8,980,319 B2 Park Et Al
US00898O319B2 (12) United States Patent (10) Patent No.: US 8,980,319 B2 Park et al. (45) Date of Patent: *Mar. 17, 2015 (54) METHODS OF PRODUCING STABILIZED A613 L/445 (2006.01) SOLID DOSAGE PHARMACEUTICAL A613 L/47 (2006.01) COMPOSITIONS CONTAINING A6II 45/06 (2006.01) MORPHINANS A63/67 (2006.01) (52) U.S. Cl. (71) Applicant: Mallinckrodt LLC, Hazelwood, MO CPC ............. A6 IK3I/485 (2013.01); A61 K9/1652 (US) (2013.01); A61 K9/2031 (2013.01); A61 K 9/2081 (2013.01); A61 K9/2086 (2013.01); (72) Inventors: Jae Han Park, Olivette, MO (US); A6IK9/2095 (2013.01); A61 K9/5042 Tiffani Eisenhauer, Columbia, IL (US); (2013.01); A61 K3I/4355 (2013.01); A61 K Spainty,S.Isna Gupta, F11llsborough, 31/4375A6 (2013.01); IK3I/445 gets (2013.01); it' A6 (2013.01); IK3I/47 Stephen Overholt, Middlesex, NJ (US) (2013.01); A61K 45/06 (2013.01); A61 K 9/2013 (2013.01); A61 K9/209 (2013.01); (73) Assignee: Mallinckrodt LLC, Hazelwood, MO A6 IK3I/167 (2013.01) (US) USPC ........... 424/472: 424/465; 424/468; 424/490; c - r - 514/282; 514/289 (*) Notice: Subject to any disclaimer, the term of this (58) Field of Classification Search patent is extended or adjusted under 35 N U.S.C. 154(b)b) by 0 daysyS. Seeone application file for complete search history. This patent is Subject to a terminal dis claimer. (56) References Cited (21) Appl. No.: 14/092.375 U.S. PATENT DOCUMENTS (22) Filed: Nov. 27, 2013 2008, 0026052 A1 ck 1/2008 Schoenhard ................. -
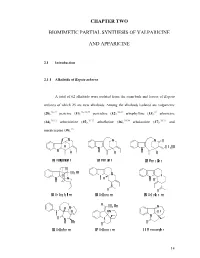
Chapter Two Biomimetic Partial Synthesis Of
CHAPTER TWO BIOMIMETIC PARTIAL SYNTHESIS OF VALPARICINE AND APPARICINE 2.1 Introduction 2.1.1 Alkaloids of Kopsia arborea A total of 62 alkaloids were isolated from the stem-bark and leaves of Kopsia arborea of which 25 are new alkaloids. Among the alkaloids isolated are valparicine (28),26,27 pericine (31),26,28,29 pericidine (32),30,31 arbophylline (33),32 arboricine (34),30,33 arboricinine (35),30,33 arboflorine (36),30,34 arboloscine (37),30,31 and mersicarpine (38).35 14 The compounds of interest to this research are valparicine (28) and pericine (31). Valparicine (28) was isolated from the stem-bark extract of K. arborea in trace amount. It represents the first member of the pericine-type alkaloids, characterized by a 16-22 exocyclic double bond, in which bond-formation has occurred between C-3 and C-7.26 Preliminary tests indicated that valparicine (28) showed strong cytotoxic effects −1 against human KB cells (IC50 < 5 μgmL ). Valparicine (28) was obtained as a colorless oil, with [α]D −40 (c 0.22, CHCl3). The UV spectrum (228 and 297 nm) indicated the presence of an unsubstituted indolenine chromophore. The EIMS of 28 showed a molecular ion at m/z 276, which 13 analyzed for C19H20N2, differing from pericine (31) by loss of two hydrogens. The C NMR spectrum gave a total of 19 carbon resonances (one methyl, five methylenes, seven methines, and six quaternary carbons) in agreement with the molecular formula. In addition to the six carbon resonances readily attributable to the aromatic moiety, and the imine resonance at δC 186.4, two other downfield quaternary resonances were observed at δC 139.2 and 144.6. -

Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders
REVIEW published: 21 August 2018 doi: 10.3389/fphar.2018.00557 Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders Maria A. Neag 1, Andrei Mocan 2*, Javier Echeverría 3, Raluca M. Pop 1, Corina I. Bocsan 1, Gianina Cri¸san 2 and Anca D. Buzoianu 1 1 Department of Pharmacology, Toxicology and Clinical Pharmacology, “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania, 2 Department of Pharmaceutical Botany, “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania, 3 Department of Environmental Sciences, Universidad de Santiago de Chile, Santiago de Chile, Chile Edited by: Berberine-containing plants have been traditionally used in different parts of the world for Anna Karolina Kiss, the treatment of inflammatory disorders, skin diseases, wound healing, reducing fevers, Medical University of Warsaw, Poland affections of eyes, treatment of tumors, digestive and respiratory diseases, and microbial Reviewed by: Pinarosa Avato, pathologies. The physico-chemical properties of berberine contribute to the high diversity Università degli Studi di Bari Aldo of extraction and detection methods. Considering its particularities this review describes Moro, Italy various methods mentioned in the literature so far with reference to the most important Sylwia Zielinska, Wroclaw Medical University, Poland factors influencing berberine extraction. Further, the common separation and detection *Correspondence: methods like thin layer chromatography, high performance liquid chromatography, and Andrei Mocan mass spectrometry are discussed in order to give a complex overview of the existing [email protected] methods. Additionally, many clinical and experimental studies suggest that berberine Specialty section: has several pharmacological properties, such as immunomodulatory, antioxidative, This article was submitted to cardioprotective, hepatoprotective, and renoprotective effects. -

Nonaceae Using Targeted Enrichment of Nuclear Genes Supplementary
Phylogenomics of the major tropical plant family An- nonaceae using targeted enrichment of nuclear genes Thomas L.P. Couvreur1,*, Andrew J. Helmstetter1, Erik J.M. Koenen2, Kevin Bethune1, Rita D. Brand~ao3, Stefan Little4, Herv´eSauquet4,5, Roy H.J. Erkens3 1 IRD, UMR DIADE, Univ. Montpellier, Montpellier, France 2 Institute of Systematic Botany, University of Zurich, Z¨urich, Switzer- land 3 Maastricht University, Maastricht Science Programme, P.O. Box 616, 6200 MD Maastricht, The Netherlands 4 Ecologie Syst´ematiqueEvolution, Univ. Paris-Sud, CNRS, AgroParis- Tech, Universit´e-Paris Saclay, 91400, Orsay, France 5 National Herbarium of New South Wales (NSW), Royal Botanic Gardens and Domain Trust, Sydney, Australia * [email protected] Supplementary Information (see next page) 1 Supplementary Table 1. Specimen details of taxa sampled for both An- nonaceae and Piptostigmateae analyses Subfamily Tribe Species Collector number Country INDEX TAG total reads Mapped % enrichment 10x coverage mean depth Ambavioideae Cleistopholis staudii Couvreur, T.L.P. 570 Gabon I12 TAG79 1790158 402150 22 0,82 119,7 Ambavioideae Drepananthus ramuliflorus Sauquet, H. 167 Malaysia I10 TAG45 2676926 150278 6 0,66 45,1 Ambavioideae Meiocarpidium olivieranum Couvreur, T.L.P. 920 Gabon I10 TAG13 3950072 343879 9 0,80 104,3 Anaxagoreoideae Anaxagorea crassipetala Maas, P.J.M. 9408 Costa Rica I10 TAG25 2648398 256748 10 0,67 76,9 Annonoideae Annoneae Annona glabra Chatrou, L.W. 467 Peru I10 TAG36 4328486 622387 14 0,83 190,7 Annonoideae Annoneae Anonidium mannii Couvreur, T.L.P. 1053 Cameroon I04 TAG36 1613002 679049 42 0,89 206,9 Annonoideae Annoneae Boutiquea platypetala Couvreur, T.L.P.