CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Reg. No Name in Full Residential Address Gender Contact No. Email Id Remarks 9421864344 022 25401313 / 9869262391 Bhaveshwarikar
Reg. No Name in Full Residential Address Gender Contact No. Email id Remarks 10001 SALPHALE VITTHAL AT POST UMARI (MOTHI) TAL.DIST- Male DEFAULTER SHANKARRAO AKOLA NAME REMOVED 444302 AKOLA MAHARASHTRA 10002 JAGGI RAMANJIT KAUR J.S.JAGGI, GOVIND NAGAR, Male DEFAULTER JASWANT SINGH RAJAPETH, NAME REMOVED AMRAVATI MAHARASHTRA 10003 BAVISKAR DILIP VITHALRAO PLOT NO.2-B, SHIVNAGAR, Male DEFAULTER NR.SHARDA CHOWK, BVS STOP, NAME REMOVED SANGAM TALKIES, NAGPUR MAHARASHTRA 10004 SOMANI VINODKUMAR MAIN ROAD, MANWATH Male 9421864344 RENEWAL UP TO 2018 GOPIKISHAN 431505 PARBHANI Maharashtra 10005 KARMALKAR BHAVESHVARI 11, BHARAT SADAN, 2 ND FLOOR, Female 022 25401313 / bhaveshwarikarmalka@gma NOT RENEW RAVINDRA S.V.ROAD, NAUPADA, THANE 9869262391 il.com (WEST) 400602 THANE Maharashtra 10006 NIRMALKAR DEVENDRA AT- MAREGAON, PO / TA- Male 9423652964 RENEWAL UP TO 2018 VIRUPAKSH MAREGAON, 445303 YAVATMAL Maharashtra 10007 PATIL PREMCHANDRA PATIPURA, WARD NO.18, Male DEFAULTER BHALCHANDRA NAME REMOVED 445001 YAVATMAL MAHARASHTRA 10008 KHAN ALIMKHAN SUJATKHAN AT-PO- LADKHED TA- DARWHA Male 9763175228 NOT RENEW 445208 YAVATMAL Maharashtra 10009 DHANGAWHAL PLINTH HOUSE, 4/A, DHARTI Male 9422288171 RENEWAL UP TO 05/06/2018 SUBHASHKUMAR KHANDU COLONY, NR.G.T.P.STOP, DEOPUR AGRA RD. 424005 DHULE Maharashtra 10010 PATIL SURENDRANATH A/P - PALE KHO. TAL - KALWAN Male 02592 248013 / NOT RENEW DHARMARAJ 9423481207 NASIK Maharashtra 10011 DHANGE PARVEZ ABBAS GREEN ACE RESIDENCY, FLT NO Male 9890207717 RENEWAL UP TO 05/06/2018 402, PLOT NO 73/3, 74/3 SEC- 27, SEAWOODS, -

Bpc(Maharashtra) (Times of India).Xlsx
Notice for appointment of Regular / Rural Retail Outlet Dealerships BPCL proposes to appoint Retail Outlet dealers in Maharashtra as per following details : Sl. No Name of location Revenue District Type of RO Estimated Category Type of Minimum Dimension (in Finance to be arranged by the applicant Mode of Fixed Fee / Security monthly Site* M.)/Area of the site (in Sq. M.). * (Rs in Lakhs) Selection Minimum Bid Deposit Sales amount Potential # 1 2 3 4 5 6 7 8 9a 9b 10 11 12 Regular / Rural MS+HSD in SC/ SC CC1/ SC CC- CC/DC/C Frontage Depth Area Estimated working Estimated fund required Draw of Rs in Lakhs Rs in Lakhs Kls 2/ SC PH/ ST/ ST CC- FS capital requirement for development of Lots / 1/ ST CC-2/ ST PH/ for operation of RO infrastructure at RO Bidding OBC/ OBC CC-1/ OBC CC-2/ OBC PH/ OPEN/ OPEN CC-1/ OPEN CC-2/ OPEN PH From Aastha Hospital to Jalna APMC on New Mondha road, within Municipal Draw of 1 Limits JALNA RURAL 33 ST CFS 30 25 750 0 0 Lots 0 2 Draw of 2 VIllage jamgaon taluka parner AHMEDNAGAR RURAL 25 ST CFS 30 25 750 0 0 Lots 0 2 VILLAGE KOMBHALI,TALUKA KARJAT(NOT Draw of 3 ON NH/SH) AHMEDNAGAR RURAL 25 SC CFS 30 25 750 0 0 Lots 0 2 Village Ambhai, Tal - Sillod Other than Draw of 4 NH/SH AURANGABAD RURAL 25 ST CFS 30 25 750 0 0 Lots 0 2 ON MAHALUNGE - NANDE ROAD, MAHALUNGE GRAM PANCHYAT, TAL: Draw of 5 MULSHI PUNE RURAL 300 SC CFS 30 25 750 0 0 Lots 0 2 ON 1.1 NEW DP ROAD (30 M WIDE), Draw of 6 VILLAGE: DEHU, TAL: HAVELI PUNE RURAL 140 SC CFS 30 25 750 0 0 Lots 0 2 VILLAGE- RAJEGAON, TALUKA: DAUND Draw of 7 ON BHIGWAN-MALTHAN -

Lycodon Flavomaculatus Wall 1907
WWW.IRCF.ORG/REPTILESANDAMPHIBIANSJOURNALTABLE OF CONTENTS IRCF REPTILES & IRCF AMPHIBIANS REPTILES • VOL &15, AMPHIBIANS NO 4 • DEC 2008 • 189 22(4):164–167 • DEC 2015 IRCF REPTILES & AMPHIBIANS CONSERVATION AND NATURAL HISTORY TABLE OF CONTENTS FEATURE ARTICLES A. Chasing New Bullsnakes (Pituophis Locality catenifer sayi) in Wisconsin: for the Elusive and On the Road to Understanding the Ecology and Conservation of the Midwest’s Giant Serpent ...................... Joshua M. Kapfer 190 . The Shared History of Treeboas (Corallus grenadensis) and Humans on Grenada: EndemicA Hypothetical Excursion ............................................................................................................................Yellow-Spotted WolfRobert W.Snake Henderson 198 RESEARCH(Lycodon ARTICLES flavomaculatus Wall 1907), . The Texas Horned Lizard in Central and Western Texas ....................... Emily Henry, Jason Brewer, Krista Mougey, and Gad Perry 204 . The Knight Anole (Anolis equestris) in Florida with ............................................. Notes Brianon J. Camposano, Distribution Kenneth L. Krysko, Kevin M. Enge, Ellen M. Donlan,and and Michael Habitat Granatosky 212 CONSERVATION ALERTVivek Sharma1, Arpit Jain2, and Rita Bhandari3 . World’s Mammals in Crisis ............................................................................................................................................................. 220 1Department. More ofThan Zoology, Mammals Government ..................................................................................................................................................................... -

Proceedings of National Seminar on Biodiversity And
BIODIVERSITY AND CONSERVATION OF COASTAL AND MARINE ECOSYSTEMS OF INDIA (2012) --------------------------------------------------------------------------------------------------------------------------------------------------------- Patrons: 1. Hindi VidyaPracharSamiti, Ghatkopar, Mumbai 2. Bombay Natural History Society (BNHS) 3. Association of Teachers in Biological Sciences (ATBS) 4. International Union for Conservation of Nature and Natural Resources (IUCN) 5. Mangroves for the Future (MFF) Advisory Committee for the Conference 1. Dr. S. M. Karmarkar, President, ATBS and Hon. Dir., C B Patel Research Institute, Mumbai 2. Dr. Sharad Chaphekar, Prof. Emeritus, Univ. of Mumbai 3. Dr. Asad Rehmani, Director, BNHS, Mumbi 4. Dr. A. M. Bhagwat, Director, C B Patel Research Centre, Mumbai 5. Dr. Naresh Chandra, Pro-V. C., University of Mumbai 6. Dr. R. S. Hande. Director, BCUD, University of Mumbai 7. Dr. Madhuri Pejaver, Dean, Faculty of Science, University of Mumbai 8. Dr. Vinay Deshmukh, Sr. Scientist, CMFRI, Mumbai 9. Dr. Vinayak Dalvie, Chairman, BoS in Zoology, University of Mumbai 10. Dr. Sasikumar Menon, Dy. Dir., Therapeutic Drug Monitoring Centre, Mumbai 11. Dr, Sanjay Deshmukh, Head, Dept. of Life Sciences, University of Mumbai 12. Dr. S. T. Ingale, Vice-Principal, R. J. College, Ghatkopar 13. Dr. Rekha Vartak, Head, Biology Cell, HBCSE, Mumbai 14. Dr. S. S. Barve, Head, Dept. of Botany, Vaze College, Mumbai 15. Dr. Satish Bhalerao, Head, Dept. of Botany, Wilson College Organizing Committee 1. Convenor- Dr. Usha Mukundan, Principal, R. J. College 2. Co-convenor- Deepak Apte, Dy. Director, BNHS 3. Organizing Secretary- Dr. Purushottam Kale, Head, Dept. of Zoology, R. J. College 4. Treasurer- Prof. Pravin Nayak 5. Members- Dr. S. T. Ingale Dr. Himanshu Dawda Dr. Mrinalini Date Dr. -

List of SETU Suvidha Kendra in State of Maharashtra
List of SETU Suvidha Kendra in State of Maharashtra Region District College SSK Incharge Sr.No Taluka College Name Address Contact No. Name Name code Name AT. POST BABHULGAON, N.H. NO.6 , TQ. & Shri Shivaji Education Society's College of Engineering and 1 Amravati Akola Akola 1116 DISTRICT- AKOLA Vikas Bhugul 9923538835 Technology, Akola CITY:AKOLA PIN:444104 ANJANGAON BARI ROAD BADNERA- Prof. Prof. Ram Meghe Institute of Technology & Research, 2 Amravati Amravati Amravati 1105 AMRAVATI CITY:AMRAVATI Shailesh 9921559515 Amravati PIN:444701 Subhashrao Dhok INFRONT OF NEMANI GODOWN, BADNERA Sipna Shikshan Prasarak Mandal College of Engineering & Rothkar Prof. 3 Amravati Amravati Amravati 1114 ROAD, AMRAVATI 9766320027 Technology, Amravati Ravi CITY:AMRAVATI PIN:444701 Shri Hanuman Vyayam Prasarak Mandals College of HVPM CAMPUS, HANUMAN VYAYAM NAGAR, Satosh 4 Amravati Amravati Amravati 1121 Engineering & AMRAVATI 7972351873 Bande Technology, Amravati CITY:AMRAVATI PIN:444605 UNIVERSITY MARDI ROAD GHATKHEDA Dr Rajendra Gode Institute of Technology & Research DR PRAMOD 5 Amravati Amravati Amravati 1123 AMRAVATI 7720002401 Amravati PATIL CITY:AMRAVATI PIN:444602 ANJANGAON BARI ROAD AMRAVATI G.H. Raisoni college of Engineering & Management, Anand 6 Amravati Amravati Amravati 1124 CITY:AMRAVATI 7972999533 Amravati Deshpande PIN:444701 Prof Ram Meghe College of Engineering and Management, NEW EXPRESS HIGHWAY, BADNERA Saurabh 7 Amravati Amravati Amravati 1128 8805049699 Badnera CITY:AMRAVATI PIN:444602 Shah 1. Tawar P. R. Pote Patil College of -

Ma Ter1als & Methods
CHAPTER III MA TER1ALS & METHODS INDEX CHAPTER - III MATERIALS AND METHODS Sr.No Description Page Nos 3.1 Study Area 51 3.2 Hydrology 51 3.3 Geology 52 3.4 Soils 52 3.5 Climate 53 3.6 Forest Types 54 3.7 Wild Animals 54 3.8 Agriculture 55 3.9 Materials Used 55 3.10 Methodology 57 3.10.1 Database Organization and Design Specifications 57 3.10.2 Database Design Specifications 57 3.10.3 Digitization of various layers and GIS Development 58 3.10.4 Analysis of satellite Images 59 3.10.5 Vegetation Classification Scheme 60 3.10.6 Satellite Data Analysis for Vegetation Classification 60 3.10.7 Forest Resource Base Calculation 61 3.10.7a Basis for Stratification of Forests 61 3.10.7b Field Survey and Data Recording 64 Tables Tables Description Page Nos Table 3.1 Geographic Area, Forest Area and Population in Study Area 51 Table 3.2 Location Details of Sample Plots in Study Area 61 Table 3.3 Sample Plot Data Collection Form 66 Maps Maps Description MapNo-3.1 Map Showing the Study Area Location MapNo-3.2 Drainage Network and Water Bodies in Study Area MapNo-3.3 Thematic Map Showing Soil Depth in Study Area Plates Plate Description Plate No. 1 Forest types in Sampling sites with Location in Lat/Long Plate No.2 Forest types in Sampling sites with Location in Lat/Long Materials and Methods CHAPTER - III MATERIALS AND METHODS 3.1.Study Area The study area is located in Pune district of Maharashtra state, India and it includes the Mulshi, Haveli talukas and Pune Municipal Corporation(PMC) areas. -
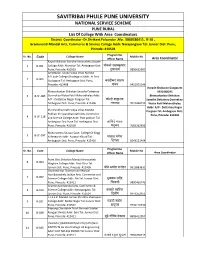
PUNE RURAL List of College with Area Coordinators District Coordinator -Dr.Shrikant Fulsundar ,Mo
SAVITRIBAI PHULE PUNE UNIVERSITY NATIONAL SERVICE SCHEME PUNE RURAL List Of College With Area Coordinators District Coordinator -Dr.Shrikant Fulsundar ,Mo. 9860286411, B-10 , Gramonnati Mandal Arts, Commerce & Science College Addr: Narayangaon Tal: Junnar Dist: Pune, Pincode: 410504 Programme Sr. No. Code College Name Mobile No Officer Name Area Coordinator Rayat Shikshan Sanstha Annasaheb Aawate 1 B-001 College Addr: Manchar Tal: Ambegaon Dist: पोकळे संजयकुमार Pune, Pincode: 410503 तुकाराम 9850652907 Ambegaon Taluka Vidya Vikas Mandal B.D.Kale College Ghodegaon Addr: At Post 2 B-005 Godegaon Tal: Ambegaon Dist: Pune, करंदीकर वभ Pincode: 412408 शंकर 9421001146 Korade Shakurao Gangaram Bhimashankar Shikshan Sanstha Dattatray 9922468295 3 B-SF-085 Govindrao Walse Patil Mahavidhylay Addr: Bhimashankar Shikshan A/P - Dattatray Nagar Pargaon Tal: कोरडे शाकुराव Sanstha Dattatray Govindrao Ambegaon Dist: Pune, Pincode: 412406 गंगाराम 9922468295 Walse Patil Mahavidhylay Addr: A/P - Dattatray Nagar Shri Pandharinath Vidya Vikas Mandal Pargaon Tal: Ambegaon Dist: Pokhari Shri pandharinath Arts Commerce Pune, Pincode: 412406 4 B-SF-118 and Science College Addr: Post pokhari Tal Ambegaon Dist Pune Tal: Ambegaon Dist: कािशदे यादव Pune, Pincode: 410509 मधुकर 7083262996 Maharashtra Shasan Govt. College Of Engg. 5 B-SF-097 & Research Addr: Awasari Khurd Tal: पांचाळ मंगेश Ambegaon Dist: Pune, Pincode: 412405 िदगंबर 8149121404 Programme Sr. No. Code College Name Mobile No Officer Name Area Coordinator Pune Jilha Shikshan Mandal Annasaheb 6 B-004 Waghire College Addr: Post-Otur Tal: Junnar Dist: Pune, Pincode: 412409 बीबे अमोल मनोहर 9623883631 Dnyaneshwar Gramonnati Mandal Hon.Balasaheb Jadhav Arts, Commerce and 7 B-007 Science College Addr: Ale Tal: Junnar Dist: भुजबळ रवीं Pune, Pincode: 412411 िचमाजी 9890460746 Gramonnati Mandal Arts, Commerce & 8 B-010 Science College Addr: Narayangaon Tal: कांबळे िल Junnar Dist: Pune, Pincode: 410504 िदलीप 8446252951 J.T.S.S.P. -

Clinical Trial Details (PDF Generation Date :- Tue, 24 Aug 2021 11:04:52 GMT)
PDF of Trial CTRI Website URL - http://ctri.nic.in Clinical Trial Details (PDF Generation Date :- Thu, 30 Sep 2021 13:17:16 GMT) CTRI Number CTRI/2009/091/000776 [Registered on: 04/05/2010] - Last Modified On Post Graduate Thesis Type of Trial Type of Study Study Design Randomized, Parallel Group, Placebo Controlled Trial Public Title of Study A phase III clinical trial for a novel herbal molecule (1% LLL-2011) developed by Lupin Li,ited which has a potential to prevent attacks of common migraine effectively. Scientific Title of A phase III, multi-center, placebo-controlled, double blind, randomized, parallel group study to Study establish the efficacy of LLL-2011 administered as a 1% nasal spray in the preventive treatment of common migraine. Secondary IDs if Any Secondary ID Identifier LRP/CTP/017/2011/III/01 Protocol Number Details of Principal Details of Principal Investigator Investigator or overall Name As per the site deta+ils Trial Coordinator (multi-center study) Designation Affiliation Address Not Applicable N/A India Phone Fax Email Details Contact Details Contact Person (Scientific Query) Person (Scientific Name Dr. Rajesh Kumawat Query) Designation Affiliation Address Lupin Limited Lupin research park, 46A/47A, Nande Village, Mulshi Taluka Pune MAHARASHTRA 411042 India Phone +91-20-66749400 Fax +91-20-66749458 Email [email protected] Details Contact Details Contact Person (Public Query) Person (Public Query) Name Dr. Saji Vijayan Designation Affiliation Address Lupin Limited Lupin research park, 46A/47A, Nande -
School Wise Result Statistics Report
MAHARASHTRA STATE BOATD OF SEC & H.SEC EDUCATION PUNE - 4 Page : 1 schoolwise performance of Fresh Regular candidates MARCH-2020 Division : PUNE Candidates passed School No. Name of the School Candidates Candidates Total Pass Registerd Appeared Pass UDISE No. Distin- Grade Grade Pass Percent ction I II Grade 11.01.001 MAHATMA GANDHI VIDYALAYA 365 365 203 130 28 3 364 99.72 27250102207 11.01.002 JANTA VIDYA MANDIR, GHODEGAON, PUNE 137 137 38 69 23 1 131 95.62 27250105704 11.01.003 HUTATMA BABU GENU VIDYALAYA,MAHALUNGE PADVAL,PUNE 95 95 58 27 5 3 93 97.89 27250104503 11.01.004 SHREE.BHAIRAVNATH VIDYALAYA,AWASARI(KH.), PUNE 90 90 49 28 11 0 88 97.77 27250101002 11.01.005 SHIVAJI VIDYALAYA, DHAMANI, PUNE 45 45 14 17 10 2 43 95.55 27250100702 11.01.006 BHIMASHANKAR VIDYAMANDIR, SHINOLI, PUNE 86 86 41 33 11 1 86 100.00 27250108104 11.01.007 VIDYA VIKAS MANDIR, AVSARI(BK), AMBEGAON, PUNE 190 190 122 56 12 0 190 100.00 27250100102 11.01.008 SHREE.MUKTADEVI VIDYALAYA, NARODI, AMBEGAON, PUNE 54 54 13 25 14 2 54 100.00 27250107403 11.01.009 SHREE.BHAIRAVANATH VIDYADHAM, LONI, PUNE 43 42 18 18 4 1 41 97.61 27250105504 11.01.010 NARSINHA VIDYALAYA, RANJANI, PUNE 183 183 63 81 30 3 177 96.72 27250105003 11.01.011 PANDIT J.N.VIDYALAYA, NIRGUDASAR, AMBEGAON, PUNE 92 92 36 35 19 2 92 100.00 27250100504 11.01.012 SHREE.WAKESHWAR VIDYALAYA, PETH, AMBEGAON, PUNE 100 100 60 23 16 0 99 99.00 27250112904 11.01.013 SHRI RAM VIDYALAYA,PIMPLGAON,KHADKI,AMBEGAON,PUNE 54 53 21 19 8 4 52 98.11 27250102603 11.01.014 SHIV SHANKAR VIDYALAYA, AMBEGAON, DIST.PUNE 24 24 6 7 10 1 24 100.00 27250110102 11.01.015 KAMALJA DEVI VIDYALAYA, KALAMB, AMBEGAON, PUNE 112 112 38 49 18 2 107 95.53 27250104104 11.01.016 SANGMESHWAR VIDYALAYA, 120 120 46 45 25 3 119 99.16 27250101903 PARGAON,(SHINGVE),DIST.PUNE 11.01.017 SANT DNYANESHWAR MAHARAJ VIDYALAYA, CHAS,AMBEGAON 58 58 35 14 9 0 58 100.00 27250107803 MAHARASHTRA STATE BOATD OF SEC & H.SEC EDUCATION PUNE - 4 Page : 2 schoolwise performance of Fresh Regular candidates MARCH-2020 Division : PUNE Candidates passed School No. -

Colleges Code Unipune.Pdf
College Code College Name City Application Count 0001 Government College of Engineering, Pune 580 0002 Fergusson College, Pune 818 0003 Gokhale Institute of Politics & Economics, Pune 6 0004 Sir Parashurambhau College, Pune 575 0005 I.L.S.'s Law College, Pune 332 0006 Nowrosjee Wadia College, Pune 442 0007 Tilak College of Education, Pune 68 0008 Brihan Maharashtra College of Commerce, Pune 413 0009 Abasaheb Garware College of Arts & Science, Pune 389 0012 Shri Shahu Mandir Mahavidyalaya, Pune 131 0013 St. Mira's College for Girls, Pune 247 0015 H.P.T. Arts & R.Y.K. Science College, Nashik 140 0016 Mooljee Jetha College, Jalgaon 3 0017 Pratap College, Amalner 3 0018 Ahmednagar College, Ahmednagar 181 0020 B.Y.K. College of Commerce, Nashik 212 0021 M.S.G. Arts, Science & Commerce College, Malegaon Camp 311 0022 R. B. Narayanrao Borawake College, Shrirampur 149 Suhrud Mandal's College of Education (Hearing 0023 Impaired), Pune 1 0028 Arts, Science & Commerce College, Chalisgaon 1 0030 S.N. Arts, D.J.M. Commerce & B.N.S. Science College, Sangamner 162 0031 Pemraj Sarda College, Ahmednagar 69 0032 Tuljaram Chaturchand College, Baramati 332 0034 C. D. Jain College of Commerce, Shrirampur 139 0036 R.N.C. Arts, J.D.B. Commerce & N.S.C. Science College, Nasik-Road 265 0038 Dada Patil Mahavidyalaya, Karjat 74 0039 K. J. Somaiya College of Arts, Commerce & Science, Kopargaon 59 S.S.G.M. Science, Gautam Arts & Sanjeevani Commerce 0042 College, Kopargaon 82 0044 College of Education, Nashik 70 0045 College of Education, Ahmednagar 27 Annasaheb Awate Arts & Commerce and Hutatma Babu 0048 Genu Science College, Manchar 64 0049 Janata Mahavidyalaya of Arts & Commerce, Pathardi 12 0052 Arts, Science & Commerce College, Satana 79 0053 N.V.P. -

LIST of COLLEGES of the UNIVERSITY Pune District Name of the University: - University of Pune Arts, Science & Commerce, B.B.A., B.C.A., B.S.C
LIST OF COLLEGES OF THE UNIVERSITY Pune District Name of the University: - University of Pune Arts, Science & Commerce, B.B.A., B.C.A., B.S.C. Sr. Distri Name of the College, Location Year of Type of Courses Whether Course Conduced No. ct Full Address with Pin Code Rural ® Establish-ment College for offered in recognize Urban Men only(M) the College d by the (U) For Women Up to UGC only(W) Bachelor under Co-education Degree Section (CE) (UG) 2(f)&12B Up to Master Degree (PG) Or above 1. 2. 3. 4. 5. 6. 7. 8. 9. 1 Pune Deccan Education Society’s U 1885 MW UG, PG B.A., B.A-Music., B.Sc., Fergusson College, B.Sc.-Computer Science, (Arts, Science) B.Sc-Bio-technology Deccan, Pune – 411 004. B.Sc-Environmental Ph.No.020-25654212/ Science, 25675960 B.Sc- Microbiology, E.Mail: [email protected] Vocational Electronic (ID.NO.PU/PN/AS/02/1885) Equipment maintenance, Vocational Still Photography and Audiovisual, Vocational Biotechnolgy M.A-English, Marathi, Economics,Psychology, M.Sc- Org Chemistry, Analytical Chemistry, Bio-Chemistry, M.Sc.Tec.Ind.Maths, C:\DOCUME~1\PC1\LOCALS~1\Temp\List on Website.docx Microbiology, Physics, Petrolium Technology, Electronics Science, Environmental science, Geology, Bio-tech, Botany, Zoology M.Sc- Computer Science MCA-(Science), 2 Pune Shikshan Prasarak Mandal’s U 1916 MW UG, PG B.A., B.Com.,B.B.A., Sir Parashurambhau College B.Sc.-Computer Science, (Arts,Science and Commerce), MA.- Economics, Politics, Tilak Road, Pune-411030. Geography, Psychology, Ph. No. 020- 24331978, Philosophy, Logic Fax No. -

Reg. No Name in Full Residential Address Gender Contact No
Reg. No Name in Full Residential Address Gender Contact No. Email id Remarks 40001 SHUKLA RAJMANI JANBHAGYODAYA CHAWL Male 022 28704928 / JAGDISHPRASAD KAMETI NANJIWADI, 9619503869 GAONDEVI RD POISAR, KANDIVALI (E) 400101 MUMBAI Maharashtra 40002 KHARABE JAYSHREE P.N. 117, ULHAS NAGAR Female 09438267463 PH. NO. 0712-2745880 SHARAD MANEWADA RD NAGPUR 440027 NAGPUR Maharashtra 40003 KEDARI SARASWATI FLAT NO.103, RIDDHI APT. Female 8983442510 / JANARDEN NEAR JONDHALE POLE TECH, 8446348463 MORIVALI PADA, AMBERNATH (E) 421501 THANE Maharashtra 40004 MUKADAM ASMA RAFI BLOCK NO. 5/A/5, ROAD NO. Female 9222161823 / 9664908761 AHMED 8 BAIGANWADI, GOVANDI, 9920870355 400043 MUMBAI Maharashtra 40005 MISTRY RASHMI BLOCK NO.7, PRATHAMESH Female 28386396 / CHANDRAKANT APT DEOLWADI, NR. SAHAR 9324906120 RD CHAKALA, ANDHERI(E) 400099 MUMBAI Maharashtra 40006 SINGH BABITA DIWAKAR FLAT NO.410, MIT NIKETAN Female 022 28701024 / TOWER OPP.90FEET ROAD, 9867396844 THAKUR COMPLEX, KANDIVLI (E) 400101 MUMBAI Maharashtra 40007 SHETTY DEEPA SANJEVA 204, RLA TOWER TANK RD Female PH. NO. 022-25952941 BHANDUP(W) MUMBAI 400078 MUMBAI MAHARASHTRA 40008 CHANDIWADE SHRIKANT A/P - LAVEL, TAL - KHED Male 9422595982 RAMCHANDRA 415708 RATNAGIRI Maharashtra 40009 BHALE MADHAVI MAHSUL COLONY, NR NEW Female 9422396506 / RENEWAL UP TO 2015 PRABHAKARRAO BUS STAND, NR JUJGAR 9422337341 HOSPITAL, MAJALGAON 431131 BEED Maharashtra 40010 POL AMOL BHASKAR A/P=DOMGAON, TAL- Male 9527213713 PARANDA DIST-OSMANABAD 413202 USMANABAD Maharashtra 40011 KASTURE SUCHITA SHARDA NAGAR, DEGLOOR Female PH. NO. 9423485733 DEFAULTER SURYAKANTRAO KRISHI DHAN NIWAS NEAR DHAGE , TAL-DEGLOOR NANDED 431717 NANDED MAHARASHTRA 40012 OMBASE MANOJ AT-DHAKANI, PO-DIWAD TAL- Male 8380802882 RAMCHANDRA MAN, 415509 SATARA Maharashtra 40013 SHIRKANDE RAHUL C/O POOJA Male 9766923235 MURLIDHAR RESIDENCY,ANAND NAGAR COLONY,NEAR NH-4 OVER BRIDGE (W), GODOLI 415001 SATARA Maharashtra 40014 GAVADE NAYANESH NITIN CLINIC, A/P - ARAWALI Male 02366 227052 / NAMADEV TAL - VENGURLA 9764593184 416518 SINDHUDURG Maharashtra 40015 HARER PADMAJA GOVT.