Methamidophos
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Malathion General Fact Sheet
MALATHION GENERAL FACT SHEET What is malathion Malathion is an insecticide in the chemical family known as organophosphates. Products containing malathion are used outdoors to control a wide variety of insects in agricultural settings and around people’s homes. Malathion has also been used in public health mosquito control and fruit fly eradication programs. Malathion may also be found in some special shampoos for treating lice. Malathion was first registered for use in the United States in 1956. What are some products that contain malathion Products containing malathion may be liquids, dusts, wettable powders, or emulsions. There are thousands of products contain- ing malathion registered for use in the United States. Always follow label instructions and take steps to avoid exposure. If any exposures occur, be sure to follow the First Aid instructions on the product label carefully. For additional treatment advice, contact the Poison Control Center at 1-800-222-1222. If you wish to discuss a pesticide problem, please call 1-800-858-7378. How does malathion work Malathion kills insects by preventing their nervous system from working properly. When healthy nerves send signals to each other, a special chemical messenger travels from one nerve to another to continue the message. The nerve signal stops when an enzyme is released into the space between the nerves. Malathion binds to the enzyme and prevents the nerve signal from stopping. This causes the nerves to signal each other without stopping. The constant nerve signals make it so the insects can’t move or breathe normally and they die. People, pets and other animals can be affected the same way as insects if they are exposed to enough malathion. -

US EPA, Pesticide Product Label, ORTHO MALATHION 50 INSECT
23V 73^ f UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 Jff***^ OFFICE OF WAV 00 Onit CHEMICAL SAFETY AND MAY 4 0 201] POLLUTION PREVENTION James Wallace The Scotts Company d/b/a The Ortho Group P.O.Box 190 Marysville, OH 43040 Subject: Submission of amended labeling per May 2009 Malathion RED Label Table EPA Reg. No. 239-739 Submissions dated March 5, 2010 Dear Mr. Wallace: The proposed labeling referred to above, submitted in connection with registration under the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA), is acceptable under FIFRA § 3(c)(5) provided that you submit two copies of your final printed label before you release the product for shipment. Products shipped after 12 months from the date of this amendment or the next printing of the label whichever occurs first, must bear the new revised label. Your release for shipment of the product bearing the amended label constitutes acceptance of these conditions. If these conditions are not complied with, the registration will be subject to cancellation in accordance with FIFRA sec. 6(e). The Basic Confidential Statement of Formula (CSF) dated 2/19/10 is acceptable. All other CSF's including Alternates have been superseded by this new CSF. This has been added to your record. Your product has met and complied with the risk mitigation provisions of the Malathion RED and therefore your product is reregistered pursuant to FIFRA § 4(g)(2)(C). But note, EPA is currently reassessing the cumulative impacts of organophosphates and that review may impact your product once complete. -

Malathion) Lotion, 0.5% Rx Only
NDC 51672-5276-4 Ovide® (malathion) Lotion, 0.5% Rx Only For topical use only. Not for oral or ophthalmic use. DESCRIPTION OVIDE Lotion contains 0.005 g of malathion per mL in a vehicle of isopropyl alcohol (78%), terpineol, dipentene, and pine needle oil. The chemical name of malathion is (±) - [(dimethoxyphosphinothioyl) - thio] butanedioic acid diethyl ester. Malathion has a molecular weight of 330.36, represented by C10H19O6PS2, and has the following chemical structure: CLINICAL PHARMACOLOGY Malathion is an organophosphate agent which acts as a pediculicide by inhibiting cholinesterase activity in vivo. Inadvertent transdermal absorption of malathion has occurred from its agricultural use. In such cases, acute toxicity was manifested by excessive cholinergic activity, i.e., increased sweating, salivary and gastric secretion, gastrointestinal and uterine motility, and bradycardia (see OVERDOSAGE). Because the potential for transdermal absorption of malathion from OVIDE Lotion is not known at this time, strict adherence to the dosing instructions regarding its use in children, method of application, duration of exposure, and frequency of application is required. INDICATIONS AND USAGE OVIDE Lotion is indicated for patients infected with Pediculus humanus capitis (head lice and their ova) of the scalp hair. CONTRAINDICATIONS OVIDE Lotion is contraindicated for neonates and infants because their scalps are more permeable and may have increased absorption of malathion. OVIDE Lotion should also not be used on individuals known to be sensitive to malathion or any of the ingredients in the vehicle. WARNINGS 1. OVIDE Lotion is flammable. The lotion and wet hair should not be exposed to open flames or electric heat sources, including hair dryers and electric curlers. -

Chlorpyrifos (Dursban) Ddvp (Dichlorvos) Diazinon Malathion Parathion
CHLORPYRIFOS (DURSBAN) DDVP (DICHLORVOS) DIAZINON MALATHION PARATHION Method no.: 62 Matrix: Air Procedure: Samples are collected by drawing known volumes of air through specially constructed glass sampling tubes, each containing a glass fiber filter and two sections of XAD-2 adsorbent. Samples are desorbed with toluene and analyzed by GC using a flame photometric detector (FPD). Recommended air volume and sampling rate: 480 L at 1.0 L/min except for Malathion 60 L at 1.0 L/min for Malathion Target concentrations: 1.0 mg/m3 (0.111 ppm) for Dichlorvos (PEL) 0.1 mg/m3 (0.008 ppm) for Diazinon (TLV) 0.2 mg/m3 (0.014 ppm) for Chlorpyrifos (TLV) 15.0 mg/m3 (1.11 ppm) for Malathion (PEL) 0.1 mg/m3 (0.008 ppm) for Parathion (PEL) Reliable quantitation limits: 0.0019 mg/m3 (0.21 ppb) for Dichlorvos (based on the RAV) 0.0030 mg/m3 (0.24 ppb) for Diazinon 0.0033 mg/m3 (0.23 ppb) for Chlorpyrifos 0.0303 mg/m3 (2.2 ppb) for Malathion 0.0031 mg/m3 (0.26 ppb) for Parathion Standard errors of estimate at the target concentration: 5.3% for Dichlorvos (Section 4.6.) 5.3% for Diazinon 5.3% for Chlorpyrifos 5.6% for Malathion 5.3% for Parathion Status of method: Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. Date: October 1986 Chemist: Donald Burright Organic Methods Evaluation Branch OSHA Analytical Laboratory Salt Lake City, Utah 1 of 27 T-62-FV-01-8610-M 1. -

Malathion Human Health and Ecological Risk Assessment Final Report
SERA TR-052-02-02c Malathion Human Health and Ecological Risk Assessment Final Report Submitted to: Paul Mistretta, COR USDA/Forest Service, Southern Region 1720 Peachtree RD, NW Atlanta, Georgia 30309 USDA Forest Service Contract: AG-3187-C-06-0010 USDA Forest Order Number: AG-43ZP-D-06-0012 SERA Internal Task No. 52-02 Submitted by: Patrick R. Durkin Syracuse Environmental Research Associates, Inc. 5100 Highbridge St., 42C Fayetteville, New York 13066-0950 Fax: (315) 637-0445 E-Mail: [email protected] Home Page: www.sera-inc.com May 12, 2008 Table of Contents Table of Contents............................................................................................................................ ii List of Figures................................................................................................................................. v List of Tables ................................................................................................................................. vi List of Appendices ......................................................................................................................... vi List of Attachments........................................................................................................................ vi ACRONYMS, ABBREVIATIONS, AND SYMBOLS ............................................................... vii COMMON UNIT CONVERSIONS AND ABBREVIATIONS.................................................... x CONVERSION OF SCIENTIFIC NOTATION .......................................................................... -

744 Hydrolysis of Chiral Organophosphorus Compounds By
[Frontiers in Bioscience, Landmark, 26, 744-770, Jan 1, 2021] Hydrolysis of chiral organophosphorus compounds by phosphotriesterases and mammalian paraoxonase-1 Antonio Monroy-Noyola1, Damianys Almenares-Lopez2, Eugenio Vilanova Gisbert3 1Laboratorio de Neuroproteccion, Facultad de Farmacia, Universidad Autonoma del Estado de Morelos, Morelos, Mexico, 2Division de Ciencias Basicas e Ingenierias, Universidad Popular de la Chontalpa, H. Cardenas, Tabasco, Mexico, 3Instituto de Bioingenieria, Universidad Miguel Hernandez, Elche, Alicante, Spain TABLE OF CONTENTS 1. Abstract 2. Introduction 2.1. Organophosphorus compounds (OPs) and their toxicity 2.2. Metabolism and treatment of OP intoxication 2.3. Chiral OPs 3. Stereoselective hydrolysis 3.1. Stereoselective hydrolysis determines the toxicity of chiral compounds 3.2. Hydrolysis of nerve agents by PTEs 3.2.1. Hydrolysis of V-type agents 3.3. PON1, a protein restricted in its ability to hydrolyze chiral OPs 3.4. Toxicity and stereoselective hydrolysis of OPs in animal tissues 3.4.1. The calcium-dependent stereoselective activity of OPs associated with PON1 3.4.2. Stereoselective hydrolysis commercial OPs pesticides by alloforms of PON1 Q192R 3.4.3. PON1, an enzyme that stereoselectively hydrolyzes OP nerve agents 3.4.4. PON1 recombinants and stereoselective hydrolysis of OP nerve agents 3.5. The activity of PTEs in birds 4. Conclusions 5. Acknowledgments 6. References 1. ABSTRACT Some organophosphorus compounds interaction of the racemic OPs with these B- (OPs), which are used in the manufacturing of esterases (AChE and NTE) and such interactions insecticides and nerve agents, are racemic mixtures have been studied in vivo, ex vivo and in vitro, using with at least one chiral center with a phosphorus stereoselective hydrolysis by A-esterases or atom. -

Aldicarb by Caroline Cox Pulses Across the Junctions Between Nerves
Aldicarb By Caroline Cox pulses across the junctions between nerves. (given over a one-year period). The high- This causes loss of muscular coordination, est dose at which no adverse effects were In 1966, four years before the insecticide convulsions, and ultimately death.5 The observed (called the NOEL) was 0.02 mg/ 11 aldicarb was registered for use in the United AChE inhibition is said to be reversible be- kg/day, about one-tenth of the LD50. States, researchers were using the chemical cause the aldicarb disassociates from the There is evidence that people may suf- on an experimental basis.1 One researcher AChE within several hours. This occurs even fer acute symptoms when exposed to even brought a small amount home and his wife if death has occurred. Organophosphate in- lower levels of aldicarb. In humans who applied it to the soil under a backyard rose secticides (malathion and diazinon, for ex- consumed aldicarb-contaminated water- bush. Over three weeks later she ate a sprig ample) have the same mode of action ex- melons, clinical signs of aldicarb poison- of mint from a plant growing nearby. Within cept that the AChE inhibition is not as ing were found in individuals consuming half an hour she was suffering from vomit- readily reversible.7 as little as 0.002 mg/kg of the aldicarb ing, diarrhea, and involuntary urination. Her Aldicarb is a systemic insecticide. It is metabolite aldicarb sulfoxide. This pupils closed to pinpoints, her muscles applied as granules below the soil surface amount is one-tenth the NOEL in the twitched, and her breathing was difficult. -

High Hazard Chemical Policy
Environmental Health & Safety Policy Manual Issue Date: 2/23/2011 Policy # EHS-200.09 High Hazard Chemical Policy 1.0 PURPOSE: To minimize hazardous exposures to high hazard chemicals which include select carcinogens, reproductive/developmental toxins, chemicals that have a high degree of toxicity. 2.0 SCOPE: The procedures provide guidance to all LSUHSC personnel who work with high hazard chemicals. 3.0 REPONSIBILITIES: 3.1 Environmental Health and Safety (EH&S) shall: • Provide technical assistance with the proper handling and safe disposal of high hazard chemicals. • Maintain a list of high hazard chemicals used at LSUHSC, see Appendix A. • Conduct exposure assessments and evaluate exposure control measures as necessary. Maintain employee exposure records. • Provide emergency response for chemical spills. 3.2 Principle Investigator (PI) /Supervisor shall: • Develop and implement a laboratory specific standard operation plan for high hazard chemical use per OSHA 29CFR 1910.1450 (e)(3)(i); Occupational Exposure to Hazardous Chemicals in Laboratories. • Notify EH&S of the addition of a high hazard chemical not previously used in the laboratory. • Ensure personnel are trained on specific chemical hazards present in the lab. • Maintain Material Safety Data Sheets (MSDS) for all chemicals, either on the computer hard drive or in hard copy. • Coordinate the provision of medical examinations, exposure monitoring and recordkeeping as required. 3.3 Employees: • Complete all necessary training before performing any work. • Observe all safety -
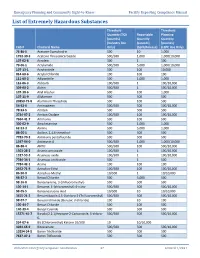
List of Extremely Hazardous Substances
Emergency Planning and Community Right-to-Know Facility Reporting Compliance Manual List of Extremely Hazardous Substances Threshold Threshold Quantity (TQ) Reportable Planning (pounds) Quantity Quantity (Industry Use (pounds) (pounds) CAS # Chemical Name Only) (Spill/Release) (LEPC Use Only) 75-86-5 Acetone Cyanohydrin 500 10 1,000 1752-30-3 Acetone Thiosemicarbazide 500/500 1,000 1,000/10,000 107-02-8 Acrolein 500 1 500 79-06-1 Acrylamide 500/500 5,000 1,000/10,000 107-13-1 Acrylonitrile 500 100 10,000 814-68-6 Acrylyl Chloride 100 100 100 111-69-3 Adiponitrile 500 1,000 1,000 116-06-3 Aldicarb 100/500 1 100/10,000 309-00-2 Aldrin 500/500 1 500/10,000 107-18-6 Allyl Alcohol 500 100 1,000 107-11-9 Allylamine 500 500 500 20859-73-8 Aluminum Phosphide 500 100 500 54-62-6 Aminopterin 500/500 500 500/10,000 78-53-5 Amiton 500 500 500 3734-97-2 Amiton Oxalate 100/500 100 100/10,000 7664-41-7 Ammonia 500 100 500 300-62-9 Amphetamine 500 1,000 1,000 62-53-3 Aniline 500 5,000 1,000 88-05-1 Aniline, 2,4,6-trimethyl- 500 500 500 7783-70-2 Antimony pentafluoride 500 500 500 1397-94-0 Antimycin A 500/500 1,000 1,000/10,000 86-88-4 ANTU 500/500 100 500/10,000 1303-28-2 Arsenic pentoxide 100/500 1 100/10,000 1327-53-3 Arsenous oxide 100/500 1 100/10,000 7784-34-1 Arsenous trichloride 500 1 500 7784-42-1 Arsine 100 100 100 2642-71-9 Azinphos-Ethyl 100/500 100 100/10,000 86-50-0 Azinphos-Methyl 10/500 1 10/10,000 98-87-3 Benzal Chloride 500 5,000 500 98-16-8 Benzenamine, 3-(trifluoromethyl)- 500 500 500 100-14-1 Benzene, 1-(chloromethyl)-4-nitro- 500/500 -

Bio-Based Reactive Diluents and Thiol-Ene
BIO-BASED REACTIVE DILUENTS AND THIOL-ENE PHOTOPOLYMERIZATION FOR ENVIRONMENTALLY BENIGN COATINGS A Dissertation Presented to The Graduate Faculty of the University of Akron In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Kosin Wutticharoenwong December, 2007 BIO-BASED REACTIVE DILUENTS AND THIOL-ENE PHOTOPOLYMERIZATION FOR ENVIRONMENTALLY BENIGN COATINGS Kosin Wutticharoenwong Dissertation Approved: Accepted: _____________________________ _____________________________ Advisor Department Chair Dr. Mark D. Soucek Dr. Sadhan C. Jana _____________________________ _____________________________ Committee Member Dean of the College Dr. Kevin Cavicchi Dr. Stephen Cheng _____________________________ _____________________________ Committee Member Dean of the Graduate School Dr. Kyonsuku Min Cakmak Dr. George R. Newkome _____________________________ _____________________________ Committee Member Date Dr. George Chase _____________________________ Committee Member Dr. Wiley J. Youngs ii ABSTRACT Tung oil was used as diene for modification via a Diels-Alder reaction with acrylate dienophiles. Tung oil was modified with three different acrylate molecules: 3- methacryloxypropyl trimethoxysilane (MAS), 2,2,2-trifluoroethyl methacrylate (TFM) and triallyl ether acrylate (TAEA) at atmospheric pressure. The modified tung oils were characterized using 1H NMR, 13C NMR, and FT-IR. The molecular weight and distribution were characterized using GPC, and MALDI-TOF. The effects of new acrylate modified tung oils on the properties of alkyd-based coatings film were investigated including hardness, solvent resistance, flexibility, gloss, impact resistance, contact angle, tensile, and thermo-mechanical properties. The viscosity can be reduced to the application viscosity of the alkyd by the reactive diluents. Drying time study showed that drying time can be altered by types and level of diluent added. All the results revealed that modified tung oils can be used as volatile organic compound (VOC) compliant in alkyd systems. -

Pesticide Residues : Maximum Residue Limits
THAI AGRICULTURAL STANDARD TAS 9002-2013 PESTICIDE RESIDUES : MAXIMUM RESIDUE LIMITS National Bureau of Agricultural Commodity and Food Standards Ministry of Agriculture and Cooperatives ICS 67.040 ISBN UNOFFICAL TRANSLATION THAI AGRICULTURAL STANDARD TAS 9002-2013 PESTICIDE RESIDUES : MAXIMUM RESIDUE LIMITS National Bureau of Agricultural Commodity and Food Standards Ministry of Agriculture and Cooperatives 50 Phaholyothin Road, Ladyao, Chatuchak, Bangkok 10900 Telephone (662) 561 2277 Fascimile: (662) 561 3357 www.acfs.go.th Published in the Royal Gazette, Announcement and General Publication Volume 131, Special Section 32ง (Ngo), Dated 13 February B.E. 2557 (2014) (2) Technical Committee on the Elaboration of the Thai Agricultural Standard on Maximum Residue Limits for Pesticide 1. Mrs. Manthana Milne Chairperson Department of Agriculture 2. Mrs. Thanida Harintharanon Member Department of Livestock Development 3. Mrs. Kanokporn Atisook Member Department of Medical Sciences, Ministry of Public Health 4. Mrs. Chuensuke Methakulawat Member Office of the Consumer Protection Board, The Prime Minister’s Office 5. Ms. Warunee Sensupa Member Food and Drug Administration, Ministry of Public Health 6. Mr. Thammanoon Kaewkhongkha Member Office of Agricultural Regulation, Department of Agriculture 7. Mr. Pisan Pongsapitch Member National Bureau of Agricultural Commodity and Food Standards 8. Ms. Wipa Thangnipon Member Office of Agricultural Production Science Research and Development, Department of Agriculture 9. Ms. Pojjanee Paniangvait Member Board of Trade of Thailand 10. Mr. Charoen Kaowsuksai Member Food Processing Industry Club, Federation of Thai Industries 11. Ms. Natchaya Chumsawat Member Thai Agro Business Association 12. Mr. Sinchai Swasdichai Member Thai Crop Protection Association 13. Mrs. Nuansri Tayaputch Member Expert on Method of Analysis 14. -

How to Reduce Bee Poisoning from Pesticides
PNW 591 December 2006 How to ReduceReduce BeeBee PoisoningPoisoning from pesticides H. Riedl E. Johansen L. Brewer J. Barbour A Pacific Northwest Extension publication Oregon State University • University of Idaho • Washington State University Contents Pollinators are essential to Pacific Northwest agriculture .......................................................................1 Rules to protect bees ..............................................................................................................................1 Causes of bee poisoning in the Pacific Northwest .................................................................................2 Investigating a suspected bee poisoning ................................................................................................2 Signs and symptoms of bee poisoning ...................................................................................................2 Honey bees .................................................................................................................................................... 2 Managed solitary bees ................................................................................................................................... 3 Ways to reduce bee poisoning ...............................................................................................................3 Beekeeper–grower cooperation ..................................................................................................................... 3 What pesticide