Illustrated Index of Plant Diseases in the State of Roraima, Brazil
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(US) 38E.85. a 38E SEE", A
USOO957398OB2 (12) United States Patent (10) Patent No.: US 9,573,980 B2 Thompson et al. (45) Date of Patent: Feb. 21, 2017 (54) FUSION PROTEINS AND METHODS FOR 7.919,678 B2 4/2011 Mironov STIMULATING PLANT GROWTH, 88: R: g: Ei. al. 1 PROTECTING PLANTS FROM PATHOGENS, 3:42: ... g3 is et al. A61K 39.00 AND MMOBILIZING BACILLUS SPORES 2003/0228679 A1 12.2003 Smith et al." ON PLANT ROOTS 2004/OO77090 A1 4/2004 Short 2010/0205690 A1 8/2010 Blä sing et al. (71) Applicant: Spogen Biotech Inc., Columbia, MO 2010/0233.124 Al 9, 2010 Stewart et al. (US) 38E.85. A 38E SEE",teWart et aal. (72) Inventors: Brian Thompson, Columbia, MO (US); 5,3542011/0321197 AllA. '55.12/2011 SE",Schön et al.i. Katie Thompson, Columbia, MO (US) 2012fO259101 A1 10, 2012 Tan et al. 2012fO266327 A1 10, 2012 Sanz Molinero et al. (73) Assignee: Spogen Biotech Inc., Columbia, MO 2014/0259225 A1 9, 2014 Frank et al. US (US) FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this CA 2146822 A1 10, 1995 patent is extended or adjusted under 35 EP O 792 363 B1 12/2003 U.S.C. 154(b) by 0 days. EP 1590466 B1 9, 2010 EP 2069504 B1 6, 2015 (21) Appl. No.: 14/213,525 WO O2/OO232 A2 1/2002 WO O306684.6 A1 8, 2003 1-1. WO 2005/028654 A1 3/2005 (22) Filed: Mar. 14, 2014 WO 2006/O12366 A2 2/2006 O O WO 2007/078127 A1 7/2007 (65) Prior Publication Data WO 2007/086898 A2 8, 2007 WO 2009037329 A2 3, 2009 US 2014/0274707 A1 Sep. -

Supplementary Table S1 18Jan 2021
Supplementary Table S1. Accurate scientific names of plant pathogenic fungi and secondary barcodes. Below is a list of the most important plant pathogenic fungi including Oomycetes with their accurate scientific names and synonyms. These scientific names include the results of the change to one scientific name for fungi. For additional information including plant hosts and localities worldwide as well as references consult the USDA-ARS U.S. National Fungus Collections (http://nt.ars- grin.gov/fungaldatabases/). Secondary barcodes, where available, are listed in superscript between round parentheses after generic names. The secondary barcodes listed here do not represent all known available loci for a given genus. Always consult recent literature for which primers and loci are required to resolve your species of interest. Also keep in mind that not all barcodes are available for all species of a genus and that not all species/genera listed below are known from sequence data. GENERA AND SPECIES NAME AND SYNONYMYS DISEASE SECONDARY BARCODES1 Kingdom Fungi Ascomycota Dothideomycetes Asterinales Asterinaceae Thyrinula(CHS-1, TEF1, TUB2) Thyrinula eucalypti (Cooke & Massee) H.J. Swart 1988 Target spot or corky spot of Eucalyptus Leptostromella eucalypti Cooke & Massee 1891 Thyrinula eucalyptina Petr. & Syd. 1924 Target spot or corky spot of Eucalyptus Lembosiopsis eucalyptina Petr. & Syd. 1924 Aulographum eucalypti Cooke & Massee 1889 Aulographina eucalypti (Cooke & Massee) Arx & E. Müll. 1960 Lembosiopsis australiensis Hansf. 1954 Botryosphaeriales Botryosphaeriaceae Botryosphaeria(TEF1, TUB2) Botryosphaeria dothidea (Moug.) Ces. & De Not. 1863 Canker, stem blight, dieback, fruit rot on Fusicoccum Sphaeria dothidea Moug. 1823 diverse hosts Fusicoccum aesculi Corda 1829 Phyllosticta divergens Sacc. 1891 Sphaeria coronillae Desm. -

CHAPTER 3 the GENUS Oxydothis and ITS PHYLOGENETIC RELATIONSHIP WITHIN the XYLARIALES 3.1. Introduction Oxydothis Penz. and Sacc
CHAPTER 3 THE GENUS Oxydothis AND ITS PHYLOGENETIC RELATIONSHIP WITHIN THE XYLARIALES 3.1. Introduction Oxydothis Penz. and Sacc. (Xylariales) includes more than 70 ascomycete species, which are saprobic, endophytic or parasitic on members of the Gramineae, Liliaceae, Palmae and Pandanaceae (Penzig and Saccardo, 1897; Hyde et al., 2000). The genus is typified by Oxydothis grisea Penz. and Sacc. (Penzig and Saccardo, 1897) and delimited based on morphological features such as long cylindrical asci with a J+ subapical apparatus; and long fusiform to filiform, hyaline, bicelled ascospores, which taper from the centre to spine±like ends, pointed or rounded processes (Penzig and Saccardo, 1897; Hyde, 1994a, b). The familial placement of Oxydothis, however, is still tentative. There is uncertainty as to which morphological characters should be used to delimit the genus as its taxonomic and phylogenetic relationships with other members of the Xylariales are still not resolved. Based on morphological similarities with Leiosphaerella Höhn., and presence of horizontal and clypeate ascomata, Oxydothis was placed within the Amphisphaeriaceae (Müller and Arx, 1962, 1976; Wehmeyer 1975; Samuels and Rossman, 1987). The genus was, however, referred to the Physosporellaceae (Phyllacorales) by Barr (1976) and later transferred to the Hyponectriaceae based on the perithecial ascomata with papillate ostiole and cylindrical asci (Hawksworth et al., 1995). A scanning Electron Microscopy (SEM) study of the ultrastructure of the 151 Oxydothis ascus could not resolve the generic and familial affiliations of Oxydothis (Wong and Hyde, 1999). Wang and Hyde (1999) excluded Oxydothis from the Hyponectriaceae based on the arrangement of the ascomata, structure of the asci and ascospores which are unlike Hyponectria buxi. -

First Record of Monographella Albescens on Rice in Corrientes Province, Argentina
CSIRO PUBLISHING www.publish.csiro.au/journals/apdn Australasian Plant Disease Notes, 2007, 2, 19–20 First record of Monographella albescens on rice in Corrientes Province, Argentina S. A. Gutierrez´ A,D,E.M.ReisB and M. A. CarmonaC ACatedra´ de Fitopatolog´ıa, Facultad de Ciencias Agrarias, Universidad Nacional del Nordeste, Sargento Cabral 2131 (3400), Corrientes, Argentina. BFitopatolog´ıa, Facultade de Agronomia e Veterinaria, UPF, Cx. Postal 611, 99001-970, Passo Fundo, Brasil. CFitopatolog´ıa, Facultad de Agronomia, Universidad de Buenos Aires, Av. San Mart´ın 4453 (1417), Ciudad de Buenos Aires, Argentina. DCorresponding author. Email: [email protected] Abstract. Monographella albescens (teleomorph) is recorded for the first time on leaves of rice in Corrientes Province, Argentina. Rice (Oryza sativa) is an important crop in Corrientes 12.5–20 × 3–3.7 μm (Fig. 3). The morphological features agree Province, Argentina. Leaf scald of rice is a disease of with those given by other authors (Ou et al.1978; Boratynski increasing importance in rice crops (Mazzanti de Castan˜on´ 1979; Parkinson et al. 1981). and Gutierrez´ 2001). The disease is characterised by zonate lesions with alternating light tan to dark brown bands that dry out giving leaves and foliar sheaths a scalded appearance (Fig. 1). The disease is caused by the fungus Monographella albescens (Thum.)¨ V.O. Parkinson, Sivan. & C. Booth (synonym Metasphaeria albescens Thum.)¨ which is usually detected in infected tissue in its anamorphic stage, Microdochium oryzae (Hashioka and Yokogi) Samuels & I.C. Hallet (synonyms Gerlachia oryzae (Hashioka and Yokogi) W. Gams and Rhynchosporium oryzae Hashioka and Yokogi). -

Role of Silicon on Plant-Pathogen Interactions. Front. Plant Sci. 8:701
fpls-08-00701 May 3, 2017 Time: 15:30 # 1 REVIEW published: 05 May 2017 doi: 10.3389/fpls.2017.00701 Role of Silicon on Plant–Pathogen Interactions Min Wang, Limin Gao, Suyue Dong, Yuming Sun, Qirong Shen and Shiwei Guo* Jiangsu Provincial Key Lab for Organic Solid Waste Utilization, National Engineering Research Center for Organic-Based Fertilizers, Jiangsu Collaborative Innovation Center for Solid Organic Waste Resource Utilization, Nanjing Agricultural University, Nanjing, China Although silicon (Si) is not recognized as an essential element for general higher plants, it has beneficial effects on the growth and production of a wide range of plant species. Si is known to effectively mitigate various environmental stresses and enhance plant resistance against both fungal and bacterial pathogens. In this review, the effects of Si on plant–pathogen interactions are analyzed, mainly on physical, biochemical, and molecular aspects. In most cases, the Si-induced biochemical/molecular resistance during plant–pathogen interactions were dominated as joint resistance, involving activating defense-related enzymes activates, stimulating antimicrobial compound production, regulating the complex network of signal pathways, and activating of the expression of defense-related genes. The most previous studies described an independent process, however, the whole plant resistances were rarely considered, especially the interaction of different process in higher plants. Si can act as a modulator influencing plant defense responses and interacting with key components of plant Edited by: Rupesh Kailasrao Deshmukh, stress signaling systems leading to induced resistance. Priming of plant defense Laval University, Canada responses, alterations in phytohormone homeostasis, and networking by defense Reviewed by: signaling components are all potential mechanisms involved in Si-triggered resistance Huixia Shou, Zhejiang University, China responses. -

Characterising Plant Pathogen Communities and Their Environmental Drivers at a National Scale
Lincoln University Digital Thesis Copyright Statement The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). This thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: you will use the copy only for the purposes of research or private study you will recognise the author's right to be identified as the author of the thesis and due acknowledgement will be made to the author where appropriate you will obtain the author's permission before publishing any material from the thesis. Characterising plant pathogen communities and their environmental drivers at a national scale A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of Philosophy at Lincoln University by Andreas Makiola Lincoln University, New Zealand 2019 General abstract Plant pathogens play a critical role for global food security, conservation of natural ecosystems and future resilience and sustainability of ecosystem services in general. Thus, it is crucial to understand the large-scale processes that shape plant pathogen communities. The recent drop in DNA sequencing costs offers, for the first time, the opportunity to study multiple plant pathogens simultaneously in their naturally occurring environment effectively at large scale. In this thesis, my aims were (1) to employ next-generation sequencing (NGS) based metabarcoding for the detection and identification of plant pathogens at the ecosystem scale in New Zealand, (2) to characterise plant pathogen communities, and (3) to determine the environmental drivers of these communities. First, I investigated the suitability of NGS for the detection, identification and quantification of plant pathogens using rust fungi as a model system. -

Long-Term Effects of Straw and Straw-Derived Biochar on Soil Aggregation and Fungal Community in a Rice&Ndash
Long-term effects of straw and straw-derived biochar on soil aggregation and fungal community in a rice–wheat rotation system Naling Bai1,2,*, Hanlin Zhang1,2,*, Shuangxi Li1,2, Xianqing Zheng1,2, Juanqin Zhang1,2, Haiyun Zhang1,2, Sheng Zhou1,2, Huifeng Sun1,2 and Weiguang Lv1,2,3 1 Eco-environmental Protection Research Institute, Shanghai Academy of Agricultural Science, Shanghai, China 2 Agricultural Environment and Farmland Conservation Experiment Station of Ministry Agriculture, Shanghai, China 3 Shanghai Key Laboratory of Protected Horticultural Technology, Shanghai, China * These authors contributed equally to this work. ABSTRACT Background: Soil aggregation is fundamental for soil functioning and agricultural productivity. Aggregate formation depends on microbial activity influencing the production of exudates and hyphae, which in turn act as binding materials. Fungi are also important for improving soil quality and promoting plant growth in a symbiotic manner. There is a scarcity of findings comparing the long-term impacts of different yearly double-crop straw return modes (e.g., straw return to the field and straw-derived biochar return to the field) on soil aggregation and fungal community structure in rice–wheat rotation systems. Methods: The effects of 6-year continuous straw and straw-derived biochar amendment on soil physicochemical properties and the fungal community were evaluated in an intensively managed crop rotation system (rice–wheat). Soil samples of different aggregates (macroaggregates, microaggregates, and silt clay) from Submitted 14 October 2018 four different fertilization regimes (control, CK; traditional inorganic fertilization, 27 November 2018 Accepted CF; straw returned to field, CS; straw-derived biochar addition, CB) were obtained, Published 4 January 2019 and Illumina MiSeq sequencing analysis of the fungal internal transcribed Corresponding author Weiguang Lv, spacer gene was performed. -
Fiji Pest Status – Attachment 3
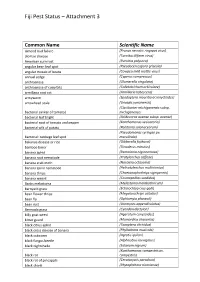
Fiji Pest Status – Attachment 3 Common Name Scientific Name almond bud failure (Prunus necrotic ringspot virus) alomae disease (Taro bacilliform virus) American corn rust (Puccinia polysora) angular bean leaf spot (Pseudocercospora griseola) angular mosaic of beans (Cowpea mild mottle virus) annual sedge (Cyperus compressus) anthracnose (Glomerella cingulata) anthracnose of cucurbits (Colletotrichum orbiculare) armillaria root rot (Armillaria tabescens) armyworm (Spodoptera mauritia acronyctoides) arrowhead scale (Unaspis yanonensis) (Clavibacter michiganensis subsp. bacterial canker of tomato) michiganensis bacterial leaf blight (Acidovorax avenae subsp. avenae) bacterial spot of tomato and pepper (Xanthomonas vesicatoria) bacterial wilt of potato (Ralstonia solanacearum) (Pseudomonas syringae pv. bacterial: cabbage leaf spot maculicola) bakanae disease or rice (Gibberella fujikuroi) bamboo borer (Dinoderus minutus) banana aphid (Pentalonia nigronervosa) banana root nematode (Pratylenchus coffeae) banana scab moth (Nacoleia octasema) banana spiral nematode (Helicotylenchus multicinctus) banana thrips (Chaetanaphothrips signipennis) banana weevil (Cosmopolites sordidus) Banks melastoma (Melastoma malabathricum) barnyard grass (Echinochloa crus-galli) bean flower thrips (Megalurothrips usitatus) bean fly (Ophiomyia phaseoli) bean rust (Uromyces appendiculatus) Bermuda grass (Cynodon dactylon) billy goat weed (Ageratum conyzoides) bitter gourd (Momordica charantia) black citrus aphid (Toxoptera citricidus) black cross disease of banana (Phyllachora -

Study of Factors Affecting Growth and Development of Narrow Brown Leaf Spot of Rice Caused by Cercospora Janseana (Racib.) O
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2015 Study of Factors Affecting Growth and Development of Narrow Brown Leaf Spot of Rice Caused by Cercospora janseana (Racib.) O. Const. kirandeep Kaur Mani Louisiana State University and Agricultural and Mechanical College, [email protected] Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Plant Sciences Commons Recommended Citation Mani, kirandeep Kaur, "Study of Factors Affecting Growth and Development of Narrow Brown Leaf Spot of Rice Caused by Cercospora janseana (Racib.) O. Const." (2015). LSU Doctoral Dissertations. 3568. https://digitalcommons.lsu.edu/gradschool_dissertations/3568 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. STUDY OF FACTORS AFFECTING GROWTH AND DEVELOPMENT OF NARROW BROWN LEAF SPOT OF RICE CAUSED BY CERCOSPORA JANSEANA (RACIB.) O. CONST. A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agricultural and Mechanical College in the partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Plant Pathology and Crop Physiology by Kirandeep Kaur Mani B.S., Punjab Agricultural University, 2005 M.S., Punjab Agricultural University, 2007 May 2015 To my parents... ii ACKNOWLEDGEMENTS I acknowledge all the people who helped me throughout my doctoral program. First of all, I am thankful to Almighty God who gave me strength and courage to finish my PhD program. -

Gerlachia Oryzae: Metasphaeria Albescens: Microdochium
11/9/2017 All data for a single taxon ***Tell us why you value the fungal databases*** Data for Microdochium albescens Microdochium albescens (Thüm.) Hern.-Restr. & Crous 2016 (Ascomycetes, Xylariales) ≡ Metasphaeria albescens Thüm. 1889 ≡Griphosphaerella albescens (Thüm.) Arx 1981 ≡Monographella albescens (Thüm.) V.O. Parkinson, Sivan. & C. Booth 1981 = Rhynchosporium oryzae Hashioka & Yokogi 1955 ≡ Microdochium oryzae (Hashioka & Yokogi) Samuels & I.C. Hallett 1983 ≡ Gerlachia oryzae (Hashioka & Yokogi) W. Gams 1980 = Micronectriella pavgii R.A. Singh 1978 Distribution: Widespread in rice-growing regions. Substrate: Living leaves; seed-borne. Disease Note: Leaf scald of rice. Host: Oryza sativa (Poaceae), reports on other grass hosts are questionable. Supporting Literature: Hernandez-Restrepo, M., Groenewald, J.Z., and Crous, P.W. 2016. Taxonomic and phylogenetic re-evaluation of Microdochium, Monographella and Idriella. Persoonia 36: 57-82. Samuels, G.J., and Hallett, I.C. 1983. Microdochium stoveri and Monographella stoveri, new combinations for Fusarium stoveri and Micronectriella stoveri. Trans. Brit. Mycol. Soc. 81: 473-483. Sivanesan, A. 1982. Monographella albescens. C.M.I. Descr. Pathog. Fungi Bact. 729: 1-2. Updated on Mar 20, 2017 Fungus-Host - 44 records were found using the criteria: name = Microdochium albescens and its synonyms Gerlachia oryzae: Oryza sativa: Korea - 39503, Metasphaeria albescens: Oryza latifolia: Venezuela - 39196, Oryza sativa Brazil - 34636,Cambodia - 24598,China - 8097, Microdochium albescens: Oryza -

Tesis Para Optar El Título De Doctor De La Universidad Nacional Del Nordeste, Area Recursos Naturales
Tesis para optar el título de Doctor de la Universidad Nacional del Nordeste, Area Recursos Naturales Monitoreo de enfermedades en semillas de arroz: detección, cuantificación y transmisión de Alternaria padwickii y Microdochium oryzae Ing. Agr. Susana Alejandra Gutiérrez Director: Ing. Agr., M. Sc., Ph.D. Erlei Melo Reis Facultade de Agronomía y Veterinaria, Universidade de Passo Fundo, Passo Fundo-Brasil Co-director: Ing. Agr. M. Sc., Marcelo Aníbal Carmona Facultad de Agronomia, Universidad de Buenos Aires, Buenos Aires AÑO 2010 AGRADECIMIENTOS Al laboratorio de la Cátedra de Fitopatología, Facultad de Ciencias Agrarias, UNNE, lugar donde se desarrolló el presente trabajo de tesis y a todo su personal docente. Al profesor Erlei, por su atención y dedicación. Al Ing. Agr. Marcelo Carmona por sus aportes y enseñanzas. A todo el personal del laboratorio de Fitopatología de la Facultad de Agronomia y Veterinaria de la Universidad de Passo Fundo (PF, Brasil) por su apoyo y colaboración desinteresada en la realización de parte del trabajo de tesis y en especial a la Bióloga Cinara Cardoso Andrade. A mi familia. 2 ABREVIATURAS ACPA Asociación Correntina de Plantadores de Arroz APD Agar papa dextrosado APG Agar papa glucosado AEM Agar extracto de malta AP Agar poroto cc Centímetros cúbicos cm Centímetros CT Control térmico DDS Días después de la siembra ET Eficiencia de transmisión FS Suspensión – Terápico para semillas g Gramos i.a. Principio activo ISTA International Seed Testing Association ha Hectárea h Horas kg Kilogramos mL Mililitros mµ Micras PG Poder germinativo PF Papel de filtro PCR Reacción en cadena de la polimerasa ppm Partes por millón R4 Embuchamiento (estado de desarrollo de la planta de arroz) SC Suspensión concentrada UV Ultravioleta v Vigor tn Toneladas TQ Tratamiento químico TT Tratamiento térmico TTQ Tratamiento térmico químico 3 INDICE PAGINAS LISTA DE CUADROS 7 LISTA DE FIGURAS 7 LISTA DE TABLAS 9 RESUMEN 11 ABSTRACT 15 CAPITULO 1. -
Checklist of Microfungi on Grasses in Thailand (Excluding Bambusicolous Fungi)
Asian Journal of Mycology 1(1): 88–105 (2018) ISSN 2651-1339 www.asianjournalofmycology.org Article Doi 10.5943/ajom/1/1/7 Checklist of microfungi on grasses in Thailand (excluding bambusicolous fungi) Goonasekara ID1,2,3, Jayawardene RS1,2, Saichana N3, Hyde KD1,2,3,4 1 Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai 57100, Thailand 2 School of Science, Mae Fah Luang University, Chiang Rai 57100, Thailand 3 Key Laboratory for Plant Biodiversity and Biogeography of East Asia (KLPB), Kunming Institute of Botany, Chinese Academy of Science, Kunming 650201, Yunnan, China 4 World Agroforestry Centre, East and Central Asia, 132 Lanhei Road, Kunming 650201, Yunnan, China Goonasekara ID, Jayawardene RS, Saichana N, Hyde KD 2018 – Checklist of microfungi on grasses in Thailand (excluding bambusicolous fungi). Asian Journal of Mycology 1(1), 88–105, Doi 10.5943/ajom/1/1/7 Abstract An updated checklist of microfungi, excluding bambusicolous fungi, recorded on grasses from Thailand is provided. The host plant(s) from which the fungi were recorded in Thailand is given. Those species for which molecular data is available is indicated. In total, 172 species and 35 unidentified taxa have been recorded. They belong to the main taxonomic groups Ascomycota: 98 species and 28 unidentified, in 15 orders, 37 families and 68 genera; Basidiomycota: 73 species and 7 unidentified, in 8 orders, 8 families and 18 genera; and Chytridiomycota: one identified species in Physodermatales, Physodermataceae. Key words – Ascomycota – Basidiomycota – Chytridiomycota – Poaceae – molecular data Introduction Grasses constitute the plant family Poaceae (formerly Gramineae), which includes over 10,000 species of herbaceous annuals, biennials or perennial flowering plants commonly known as true grains, pasture grasses, sugar cane and bamboo (Watson 1990, Kellogg 2001, Sharp & Simon 2002, Encyclopedia of Life 2018).