Wet Side Safety Manual Index
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Propoxur United States Environmental Protection Agency
United States Prevention, Pesticides EPA738-R-97-009 Environmental Protection And Toxic Substances August 1997 Agency (7508W) Reregistration Eligibility Decision (RED) PROPOXUR UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 OFFICE OF PREVENTION, PESTICIDES AND TOXIC SUBSTANCES CERTIFIED MAIL Dear Registrant: I am pleased to announce that the Environmental Protection Agency has completed its reregistration eligibility review and decisions on the pesticide chemical case propoxur. The enclosed Reregistration Eligibility Decision (RED) contains the Agency's evaluation of the data base of this chemical, its conclusions of the potential human health and environmental risks of the current product uses, and its decisions and conditions under which these uses and products will be eligible for reregistration. The RED includes the data and labeling requirements for products for reregistration. It may also include requirements for additional data (generic) on the active ingredient to confirm the risk assessments. To assist you with a proper response, read the enclosed document entitled "Summary of Instructions for Responding to the RED." This summary also refers to other enclosed documents which include further instructions. You must follow all instructions and submit complete and timely responses. The first set of required responses is due 90 days from the receipt of this letter. The second set of required responses is due 8 months from the date of receipt of this letter. Complete and timely responses will avoid the Agency taking the enforcement action of suspension against your products. If you have questions on the product specific data requirements or wish to meet with the Agency, please contact the Special Review and Reregistration Division representative Bonnie Adler (703) 308-8523. -

Regulated Substance List
INSTRUCTIONS FOR THE UNIFIED PROGRAM (UP) FORM REGULATED SUBSTANCE LIST CHEMICAL NAME CAS # TQ Listing CHEMICAL NAME CAS # TQ Listing (Lbs) Basis (Lbs) Basis Acetaldehyde 75-07-0 10,000 g Cantharidin 56-25-7 100/10,0001 * Acetone Cyanohydrin 75-86-5 1,000 Carbachol Chloride 51-83-2 500/10,0001 Acetone Thiosemicarbazide 1752-30-3 1,000/10,0001 Acetylene (Ethyne) 74-86-2 10,000 f Carbamic Acid, Methyl-,o- Acrolein (2-Propenal) 107-02-8 500 b (((2,4-Dimethyl-1,3-Dithiolan- Acrylamide 79-06-1 1,000/10,0001 2-YL) Methylene)Amino)- 26419-73-8 100/10,0001 Acrylonitrile (2- Propenenitrile) 107-13-1 10,000 b Carbofuran 1563-66-2 10/10,0001 Acrylyl Chloride Carbon Disulfide 75-15-0 10,000 b (2-Propenoyl Chloride) 814-68-6 100 b Carbon Oxysulfide Aldicarb 116-06-3 100/10,0001 (Carbon Oxide Sulfide (COS)) 463-58-1 10,000 f Aldrin 309-00-2 500/10,0001 Chlorine 7782-50-5 100 a,b Allyl Alcohol (2-Propen-1-ol) 107-18-6 1,000 b Chlorine Dioxide Allylamine (2-Propen-1-Amine) 107-11-9 500 b (Chlorine Oxide (ClO2)) 10049-04-4 1,000 c Aluminum Phosphide 20859-73-8 500 Chlorine Monoxide (Chlorine Oxide) 7791-21-1 10,000 f Aminopterin 54-62-6 500/10,0001 Chlormequat Chloride 999-81-5 100/10,0001 Amiton Oxalate 3734-97-2 100/10,0001 Chloroacetic Acid 79-11-8 100/10,0001 Ammonia, Anhydrous 2 7664-41-7 500 a,b Chloroform 67-66-3 10,000 b Ammonia, Aqueous Chloromethyl Ether (conc 20% or greater) 7664-41-7 20,000 a,b (Methane,Oxybis(chloro-) 542-88-1 100 b * Aniline 62-53-3 1,000 Chloromethyl Methyl Ether Antimycin A 1397-94-0 1,000/10,0001 (Chloromethoxymethane) -

Theoretical Study of Sarin Adsorption On
Chemical Physics Letters 738 (2020) 136816 Contents lists available at ScienceDirect Chemical Physics Letters journal homepage: www.elsevier.com/locate/cplett Research paper Theoretical study of sarin adsorption on (12,0) boron nitride nanotube doped with silicon atoms T ⁎ ⁎ Jeziel Rodrigues dos Santosa, , Elson Longo da Silvab, Osmair Vital de Oliveirac, , José Divino dos Santosa a Universidade Estadual de Goiás, Campus Anápolis, CEP: 75.132-903 GO, Brazil b INCTMN, LIEC, Departamento de Química da Universidade Federal de São Carlos, CEP: 13.565-905 São Carlos, SP, Brazil c Instituto Federal de Educação, Ciência e Tecnologia de São Paulo, Campus Catanduva, CEP: 15.808-305 Catanduva, SP, Brazil HIGHLIGHTS • DFT method was used to study the adsorption of nerve agent sarin by BNNT. • Electronic properties of pristine BNNT are improved by Si impurity atoms. • The adsorption of sarin by Si-doped BNNT is highest favorable than the pure BNNT. • Si-doped BNNT can be a new gas sensor for sarin gas detection and its derivatives. ARTICLE INFO ABSTRACT Keywords: Sarin gas is one of the most lethal nerve agent used in chemical warfare, which its detection is import to prevent Nerve agent sarin a chemical attack and to identify a contamination area. Herein, density functional theory was used to investigate Gas sensor the (12,0) boron nitride nanotube (BNNT) and Si–doped BNNT as possible candidates to sarin detection. The Si- Boron nitride nanotube atoms doped improve the electronic properties of nanotubes by altering the electrostatic potential, HOMO and DFT LUMO energies. Based in the adsorption energies and the conductivity increased to ~33 and 350%, respectively, for Si- and 2Si-BNNT imply that they can be used for sarin detection. -

Belize Pesticides Control Act Chapter 216 Revised Edition
BELIZE PESTICIDES CONTROL ACT CHAPTER 216 REVISED EDITION 2000 SHOWING THE LAW AS AT 31ST DECEMBER, 2000 This is a revised edition of the law, prepared by the Law Revision Commissioner under the authority of the Law Revision Act, Chapter 3 of the Laws of Belize, Revised Edition 1980 - 1990. This edition contains a consolidation of the following laws- Page ARRANGEMENT OF SECTIONS 3 PESTICIDES CONTROL ACT 5 Amendments in force as at 31st December, 2000. BELIZE PESTICIDES CONTROL ACT CHAPTER 216 REVISED EDITION 2000 SHOWING THE LAW AS AT 31ST DECEMBER, 2000 This is a revised edition of the law, prepared by the Law Revision Commissioner under the authority of the Law Revision Act, Chapter 3 of the Laws of Belize, Revised Edition 1980 - 1990. This edition contains a consolidation of the following laws- Page ARRANGEMENT OF SECTIONS 3 PESTICIDES CONTROL ACT 5 Amendments in force as at 31st December, 2000. Pesticides Control [CAP. 216 3 CHAPTER 216 PESTICIDES CONTROL ARRANGEMENT OF SECTIONS 1. Short title. 2. Interpretation. 3. Establishment of Pesticides Control Board. 4. Functions of the Board. 5. Policy directions. 6. Registered pesticides. 7. Licensing of manufacturer or importer of pesticides. 8. Restricted pesticides, authorisation to sell restricted pesticides and regis- tration of premises. 9. Authorisation of applicators. 10. Duties of the Secretary. 11. Correction of registers. 12. Suspension and deletion of entry from registers. 13. Prohibited pesticides. 14. Packaging. THE SUBSTANTIVE LAWS OF BELIZE REVISED EDITION 2000 Printed by the Government Printer, No. 1 Power Lane, Belmopan, by the authority of []the Government of Belize. 4 CAP. -

Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries
Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries. Peter Jentsch Extension Associate Department of Entomology Cornell University's Hudson Valley Lab 3357 Rt. 9W; PO box 727 Highland, NY 12528 email: [email protected] Phone 845-691-7151 Mobile: 845-417-7465 http://www.nysaes.cornell.edu/ent/faculty/jentsch/ 2 Historical Perspectives on Fruit Production: Fruit Tree Pest Management, Regulation and New Chemistries. by Peter Jentsch I. Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 Synthetic Pesticide Development and Use II. Influences Changing the Pest Management Profile in Apple Production Chemical Residues in Early Insect Management Historical Chemical Regulation Recent Regulation Developments Changing Pest Management Food Quality Protection Act of 1996 The Science Behind The Methodology Pesticide Revisions – Requirements For New Registrations III. Resistance of Insect Pests to Insecticides Resistance Pest Management Strategies IV. Reduced Risk Chemistries: New Modes of Action and the Insecticide Treadmill Fermentation Microbial Products Bt’s, Abamectins, Spinosads Juvenile Hormone Analogs Formamidines, Juvenile Hormone Analogs And Mimics Insect Growth Regulators Azadirachtin, Thiadiazine Neonicotinyls Major Reduced Risk Materials: Carboxamides, Carboxylic Acid Esters, Granulosis Viruses, Diphenyloxazolines, Insecticidal Soaps, Benzoyl Urea Growth Regulators, Tetronic Acids, Oxadiazenes , Particle Films, Phenoxypyrazoles, Pyridazinones, Spinosads, Tetrazines , Organotins, Quinolines. 3 I Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 The apple has a rather ominous origin. Its inception is framed in the biblical text regarding the genesis of mankind. The backdrop appears to be the turbulent setting of what many scholars believe to be present day Iraq. -

Ni33nh3+3Nhi
Pure & Appi. Chem., Vol. 49, pp.67—73. Pergamon Press, 1977. Printed in Great Britain. NON-AQJEOUS SOLVENTS FOR PREPARATION AND REACTIONS OF NITROGEN HALOGEN COMPOUNDS Jochen Jander Department of Chemistry, University of Heidelberg, D 69 Heidelberg, Germany Abstract -Theimportance of non—aqueous solvents for the chemistry of haloamines is shown in more recent reaction examples. The solvents must be low-melting because of the general temperature sensitivity of the haloamines. They may take part in the reaction (formation of CH NI •CH NH (CH )2NI, NI-,.5 NH-, and NH0I.NH-)ormay e ner (ormation of I .quinuiilidin, 2 NI •1 trithylenediamine, [I(qunuclidine),] salts pure NBr, and compounds of the type R1R2NC103 with R1 and R2 =orgnicgroup or hydrogen). INTRODUCTION Non-aqueous solvents play an important role in preparation and reactions of nitrogen halogen compounds. Due to the fact, that all haloamines are derivatives of ammonia or amines, liquid ammonia or liquid organic amines are used as solvents very often. Liquid ammonia and the lower organic amines reveal, in addition, the advantage of low melting points (liquid ammonia -78, liquid monomethylamine -93.5, liquid dimethylamine -92, liquid tn-. methylamine -12k°C); these meet the general temperature sensitivity of the nitrogen halogen compounds, especially of the pure .lorganic ones. Ammonia and amines are very seldom inert solvents; they mostly take part in the reaction planned for synthesis or conversion of the haloamine. In case a reaction of the solvent is to be avoided, one has to look for a low melting inert solvent, for example methylene chloride (m.p. -

Chemical Hygiene Plan Manual
CHEMICAL HYGIENE PLAN AND HAZARDOUS MATERIALS SAFETY MANUAL FOR LABORATORIES This is the Chemical Hygiene Plan specific to the following areas: Laboratory name or room number(s): ___________________________________ Building: __________________________________________________________ Supervisor: _______________________________________________________ Department: _______________________________________________________ Telephone numbers 911 for Emergency and urgent consultation 48221 Police business line 46919 Fire Dept business line 46371 Radiological and Environmental Management Revisied on: Enter a revision date here. All laboratory chemical use areas must maintain a work-area specific Chemical Hygiene Plan which conforms to the requirements of the OSHA Laboraotry Standard 29 CFR 19190.1450. Purdue University laboratories may use this document as a starting point for creating their work area specific CHP. Minimally this cover page is to be edited for work area specificity (non-West Lafayette laboratories are to place their own emergency, fire, and police telephone numbers in the space above) AND appendix K must be completed. This instruction and information box should remain. This model CHP is version 2010A; updates are to be found at www.purdue.edu/rem This page intentionally blank. PURDUE CHEMICAL HYGIENE PLAN AWARENESS CERTIFICATION For CHP of: ______________________________ Professor, building, rooms The Occupational Safety and Health Administration (OSHA) requires that laboratory employees be made aware of the Chemical Hygiene Plan at their place of employment (29 CFR 1910.1450). The Purdue University Chemical Hygiene Plan and Hazardous Materials Safety Manual serves as the written Chemical Hygiene Plan (CHP) for laboratories using chemicals at Purdue University. The CHP is a regular, continuing effort, not a standby or short term activity. Departments, divisions, sections, or other work units engaged in laboratory work whose hazards are not sufficiently covered in this written manual must customize it by adding their own sections as appropriate (e.g. -

University of Southampton Research Repository Eprints Soton
University of Southampton Research Repository ePrints Soton Copyright © and Moral Rights for this thesis are retained by the author and/or other copyright owners. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the copyright holder/s. The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the copyright holders. When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given e.g. AUTHOR (year of submission) "Full thesis title", University of Southampton, name of the University School or Department, PhD Thesis, pagination http://eprints.soton.ac.uk UNIVERSITY OF SOUTHAMPTON FACULTY OF SCIENCE DEPARTMENT OF CHEMISTRY Doctor of Philosophy CHEMICAL AND ELECTROCHEMICAL NITRATIONS OF ALKENES AND DIENES by Andrew Jonathan Bloom ACKNOWLEDGEMENT I wish to thank the following: My parents for their support; Dr. John Mellor and Professor Martin Fleischmann, my supervisors; Dr. Philip Parsons for his faith and encouragement; Neil Smith, Ivan Pinto, Jeff Buchannan and Neil Carlson for the proof-reading and Suzanne Salt for the typing. Financial support from The Procurement Executive, Ministry of Defence, for my maintenance grant, and NATO, for travel expenses, is gratefully acknowledged. Table of Contents Chapter 1 Introduction 1 I'l Electrochemical -
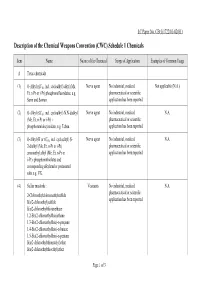
Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals
LC Paper No. CB(1)1722/01-02(01) Description of the Chemical Weapons Convention (CWC) Schedule 1 Chemicals Item Name Nature of the Chemical Scope of Application Examples of Common Usage A Toxic chemicals (1) O-Alkyl (≤C10, incl. cycloalkyl) alkyl (Me, Nerve agent No industrial, medical, Not applicable (N.A.) Et, n-Pr or i-Pr) phosphonofluoridates, e.g. pharmaceutical or scientific Sarin and Soman. application has been reported. (2) O-Alkyl (≤C10, incl. cycloalkyl) N,N-dialkyl Nerve agent No industrial, medical, N.A. (Me, Et, n-Pr or i-Pr) - pharmaceutical or scientific phosphoramidocyanidate, e.g. Tabun. application has been reported. (3) O-Alkyl (H or ≤C10, incl. cycloalkyl) S- Nerve agent No industrial, medical, N.A. 2-dialkyl (Me, Et, n-Pr or i-Pr) pharmaceutical or scientific aminoethyl alkyl (Me, Et, n-Pr or application has been reported. i-Pr)- phosphonothiolates and corresponding alkylated or protonated salts e.g. VX. (4) Sulfur mustards : Vesicants No industrial, medical, N.A. pharmaceutical or scientific 2-Chloroethylchloromethylsulfide application has been reported. Bis(2-chloroethyl)sulfide Bis(2-chloroethylthio)methane 1,2-Bis(2-chloroethylthio)ethane 1,3-Bis(2-chloroethylthio)-n-propane 1,4-Bis(2-chloroethylthio)-n-butane 1,5-Bis(2-chloroethylthio)-n-pentane Bis(2-chloroethylthiomethyl)ether Bis(2-chloroethylthioethyl)ether Page 1 of 3 Item Name Nature of the Chemical Scope of Application Examples of Common Usage (5) Lewisites : Vesicants No industrial, medical, N.A. pharmaceutical or scientific Lewisite 1 : 2-Chlorovinyldichloroarsine application has been reported. Lewisite 2 : Bis(2-chlorovinyl)chloroarsine Lewisite 3 : Tris(2-chlorovinyl)arsine (6) Nitrogen mustards : Vesicants The chemical has medical Only HN2 has been reported to application. -

Organophosphate Poisoning : a Review
120 Sinha and Sharma Med J Indones Organophosphate poisoning : A review Parmod K. Sinha, Ashok Sharma Abstrak Pestisida organofosfat digunakan secara luas di seluruh dunia. Keracunan oleh bahan ini merupakan masalah kesehatan masyarakat, terutama di negara berkembang. Zat neurotoksik organofosfat merupakan bahan yang dianggap mengancam dalam bidang militer dan terorisme. Mekanisme toksisitas bahan ini adalah dengan cara menghambat asetilkolinesterase yang mengakibatkan menumpuknya neurotransmitor asetilkolin dan terjadi rangsangan terus-menerus pada reseptor asetilkolin pada sistem saraf sentral maupun perifer. Selain krisis kolinergik, organofosfat dapat menimbulkan berbagai sindrom neurologis, baik akut maupun kronik. Sedangkan gejala peralihan ( intermediate) terjadi 1-4 hari setelah krisis kolinergik teratasi. Pengobatan standar terdiri dari reaktivasi asetilkolinesterase dengan antidot golongan oksim (prolidoksim, oksidoksime, HI-6 dan HLo7), dan pengendalian efek biokimia asetilkolin dengan menggunakan atropin. Golongan oksim yang baru HI-6 dan Hlo7 merupakan reaktivator asetilkolinesterase yang lebih cocok dan efektif untuk keracunan akut dan berat dibandingkan dengan prolidoksim dan obidoksim. Penderita yang mendapat pengobatan segera, biasanya dapat sembuh dari toksisitas akut, namun gejala neurologis ikutan dapat saja terjadi. (Med J Indones 2003; 12: 120-6) Abstract Organophosphate pesticides are used extensively worldwide, and poisoning by these agents, particularly in developing nations is a public health problem. Organophosphorous -

Theoretical Study on the Molecular and Crystal Structures of Nitrogen Trifluoride and It’S Adduct with BF3
J. Chem. Sci. Vol. 127, No. 8, August 2015, pp. 1491–1496. c Indian Academy of Sciences. DOI 10.1007/s12039-015-0857-3 Theoretical study on the molecular and crystal structures of nitrogen trifluoride and it’s adduct with BF3 HONGCHEN DUa,b,c aSchool of Science, Zhejiang A & F University, Linan, 311300, China bZhejiang Provincial Key Laboratory of Chemical Utilization of Forestry Biomass, Zhejiang A & F University, Lin’an, 311300, China cPresent address: Weifang University of Science and Technology, Jinguang Street 1299, Shouguang, 262700, China e-mail: [email protected]; [email protected] MS received 30 April 2014; revised 30 October 2014; accepted 27 January 2015 Abstract. The molecular and crystal structure of the adduct NF3·BF3 was studied computationally using density functional theory. It shows that the adduct exists in the form of a complex but is not ionic. The heats of formation in the gas and the condensed phase of the adduct are −1266.09 and −1276.37 kJ·mol−1, respectively, which indicates that it is stable under atmospheric conditions. The crystal form belongs to P 21/c space group. The calculated large band gap (Eg) of the crystal proves that it is stable. The conduction band (LUCO) is mainly contributed by the p orbital of N atom and the valence band (HOCO) from the p orbital of F atom. Keywords. Molecular; crystal; structure; property; theoretical study. 1. Introduction investigation even though there is limited information on the structure–property relationship, especially on the Molecular complexes containing boron trifluoride as a crystal structure of NF3 with BF3. -

"The Science for Diplomats" Annex on Chemicals
ORGANISATION FOR THE PROHIBITION OF CHEMICAL WEAPONS "THE SCIENCE FOR DIPLOMATS" ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals OPCW THE “SCIENCE FOR DIPLOMATS” ANNEX ON CHEMICALS A user friendly and scientifically annotated version of the Chemical Weapons Convention Annex on Chemicals1 CONTENTS A. GUIDELINES FOR SCHEDULES OF CHEMICALS B. VISUALISING AND READING MOLECULAR STRUCTURES C. SCHEDULES OF CHEMICALS D. RIOT CONTROL AGENTS 1 An official version of the Annex on Chemicals can be obtained from the OPCW public website, www.opcw.org/chemical-weapons-convention/annexes/annex-chemicals/annex-chemicals. Version 3.0 – 10 March 2019 A. GUIDELINES FOR SCHEDULES OF CHEMICALS Guidelines for Schedule 1 1. The following criteria shall be taken into account in considering whether a toxic chemical or precursor should be included in Schedule 1: (a) It has been developed, produced, stockpiled or used as a chemical weapon as defined in Article II; (b) It poses otherwise a high risk to the object and purpose of this Convention by virtue of its high potential for use in activities prohibited under this Convention because one or more of the following conditions are met: (i) It possesses a chemical structure closely related to that of other toxic chemicals listed in Schedule 1, and has, or can be expected to have, comparable properties; (ii) It possesses such lethal or incapacitating toxicity as well as other properties that would enable it to be used as a chemical weapon; (iii) It may be used as a precursor in the final single technological stage of production of a toxic chemical listed in Schedule 1, regardless of whether this stage takes place in facilities, in munitions or elsewhere; (c) It has little or no use for purposes not prohibited under this Convention.