The Krüppel-Like Factor Dar1 Determines Multipolar Neuron
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neurons and Glia
CHAPTER TWO Neurons and Glia INTRODUCTION THE NEURON DOCTRINE The Golgi Stain Cajal’s Contribution BOX 2.1 OF SPECIAL INTEREST: Advances in Microscopy THE PROTOTYPICAL NEURON The Soma The Nucleus Neuronal Genes, Genetic Variation, and Genetic Engineering BOX 2.2 BRAIN FOOD: Expressing One’s Mind in the Post-Genomic Era BOX 2.3 PATH OF DISCOVERY: Gene Targeting in Mice, by Mario Capecchi Rough Endoplasmic Reticulum Smooth Endoplasmic Reticulum and the Golgi Apparatus The Mitochondrion The Neuronal Membrane The Cytoskeleton Microtubules BOX 2.4 OF SPECIAL INTEREST: Alzheimer’s Disease and the Neuronal Cytoskeleton Microfilaments Neurofilaments The Axon The Axon Terminal The Synapse Axoplasmic Transport BOX 2.5 OF SPECIAL INTEREST: Hitching a Ride with Retrograde Transport Dendrites BOX 2.6 OF SPECIAL INTEREST: Intellectual Disability and Dendritic Spines CLASSIFYING NEURONS Classification Based on Neuronal Structure Number of Neurites Dendrites Connections Axon Length Classification Based on Gene Expression BOX 2.7 BRAIN FOOD: Understanding Neuronal Structure and Function with Incredible Cre GLIA Astrocytes Myelinating Glia Other Non-Neuronal Cells CONCLUDING REMARKS 23 © Jones & Bartlett Learning, LLC. NOT FOR SALE OR DISTRIBUTION. 24 PART ONE FOUNDATIONS INTRODUCTION All tissues and organs in the body consist of cells. The specialized func- tions of cells and how they interact determine the functions of organs. The brain is an organ—to be sure, the most sophisticated and complex organ that nature has devised. But the basic strategy for unraveling its functions is no different from that used to investigate the pancreas or the lung. We must begin by learning how brain cells work individually and then see how they are assembled to work together. -
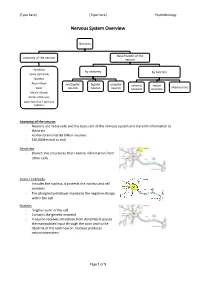
Nervous System Overview
[Type here] [Type here] Psychobiology Nervous System Overview Neurons classification of the anatomy of the neuron neuron dendrites by anatomy by function soma /cell body Nucleus Axon Hillock multipolar bipolar unipolar sensory motor interneurons Axon neuron neuron neuron neurons neurons Mylein Sheath Nodes of Ranvier Axon terminal / terminal buttons Anatomy of the neuron - Neurons are nerve cells and the basic unit of the nervous system and transmit information to the brain - Human brains has 86 billion neurons - 160,000km end to end Dendrites - Branch like structures that receive information from other cells Soma / Cell body - Includes the nucleus, it protects the nucleus and cell contents - The phospholipid bilayer maintains the negative charge within the cell Nucleus - ‘engine room’ of the cell - Contains the genetic material - If neuron receives simulation from dendrites it passes the manipulated input through the axon and to the dendrite of the next neuron. Nucleus produces neurotransmitters Page 1 of 5 [Type here] [Type here] Psychobiology Axon Hillock - The gatekeeper of transmission: this is where it is decided whether or not action potential is fired Axon terminals/ terminal buttons - Chemical messages are sent from these terminals - Gap between neurons are called synapses. Axon terminals are considered ‘pre-synaptic’ and dendrites are ‘post-synaptic’ Axon - Long nerve fibre - Transmits information to other neurons - Conducts the electrical signals from the cell body Myelin sheath - Coating that insulates the axon, composed of primarily of lipids (fats) - Allows for faster signalling - Produced by Schwan cells - Myelinated axons give some portions of the brain a white appearance Nodes of Ranvier - Bare axon - Allows the transmission to continue down the axon Classification of Neuron by Anatomy Multipolar Neuron Bipolar Neuron Unipolar Neuron - Long axon and lots of - 2 extensions from - 1 extension from the dendrites cell body cell body - (i.e. -
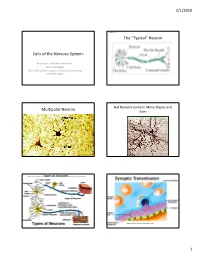
Cells of the Nervous System: the “Typical” Neuron Multipolar Neuron
2/1/2010 Book Fig. 1.1 The “Typical” Neuron Cells of the Nervous System: Neurons: cells that receive & send messages Glia: cells which support neuron functioning in many ways But Neurons Come in Many Shapes and Multipolar Neuron Sizes Types of Neurons Book Fig 1.1 Sensory Neuron Motor Neuron Some proteins serve as receptor sites. 1 2/1/2010 Best Known Neurotransmitters (study handout linked to syllabus) • Acetylcholine (ACh) • Norepinephrine (NE) • Dopamine (DA) • Serotonin or 5-Hydroxytryptamine (5HT) • GABA Released neurotransmitter must bind to specially shaped receptors like a key fitting into a lock. We now know there are multiple subtypes of receptors for each • Glutamate-most widespread excitatory neurotransmitter. transmitter Then the transmitter must be removed from the synapse either by reuptake or enzymatic breakdown. Here’s some background on ACh before we cover an Best Known Neurotransmitters Example of a neurotransmitter related disorder (FYI only – not completely up-to-date list of the number Acetylcholine (ACh) of identified receptor subtypes) • Acetylcholine (ACh) (7 receptor subtypes) • neurons using ACh are known as “cholinergic neurons”. • Examples: Norepinephrine (NE) (11 receptor subtypes) • motor neurons • Dopamine DA) (5 receptor subtypes) • parasympathetic neurons • many CNS neurons (in cortex, basal ganglia, hippocampus, • Serotonin (5HT) (14 receptor subtypes) brainstem) • GABA (2 receptor subtypes) • Different ACh receptor types on muscle (nicotinic) than in the nervous system (muscarinic) • Glutamate (10 receptor -

NERVOUS SYSTEM : LEC / 8 Physiology of Nerves and Muscles the Nervous System Is One of the Most Complicated System of the Body in Both Structure and Function
Medical physiology Lecturer Second Stage Hiba Hazim Saleh NERVOUS SYSTEM : LEC / 8 Physiology of nerves and muscles The nervous system is one of the most complicated system of the body in both structure and function. It senses physical and chemical changes in the internal and external environment, processes them, and then responds to maintain homeostasis. Voluntary activities, such as walking and talking, and involuntary activities, such as digestion and circulation, are coordinates, regulated, and integrated by the nervous system. The entire neural network of the body relies on the transmission of nervous impulses. Nervous impulses are electrochemical stimuli that travel from cell to cell as they send information from one area of the body to another. The speed at which this occurs is almost instantaneous, thus providing an immediate response change. (Figure -1. ) Muscles Muscle tissue is composed of contractile cells or fibers that provide movement of an organ or body part. Muscles contribute to posture, produce body heat, and act as a protective covering for internal organs. Muscles make up the bulk of the body. They have the ability to be excited by a stimulus, contract, relax, and return to their original size and shape. Whether muscles are attached to bones or to internal organs and blood vessels, their primary responsibility is movement. Apparent motion provided by muscles include walking and talking. Less apparent motion include the passage and elimination of food through the digestive system, propulsion of blood through the arteries, and contraction of the bladder to eliminate urine. (Figure -2 .) Figure -1 Figure-2 Never Cell (Neuron): Nerve Cell: Is a basic unit of nervous system. -

The Unipolar Neuron
The Unipolar Neuron The unipolar neuron is a sensory neuron and carries an electrical signal to the CNS. However, the anatomy of unipolar neurons is subject to different interpretations. As the unipolar neuron develops, there is a single protoplasmic process which extends from the neuron’s soma. This process splits immediately into two segments: 1) a proximal segment which enters the spinal cord; 2) a distal segment which extends out into the body and terminates in tissue as a “receptor”. Some refer to the two processes as “axons” while others refer to only the proximal process as an axon and the distal process as the dendrite. Saladin chooses the former while others (including myself!) choose the latter interpretation. Here is the critical issue. The receptor (i.e. dendrite) in the target tissue is stimulated and generates an action potential that moves towards the spinal cord (either in the dendrite or axon depending on which interpretation you choose). As the electrical signal approaches the soma, it does not need to create a local potential within the soma and the action potential continues to propagate the signal beyond the protoplasmic process of the soma. At the point of the soma’s protoplasmic process, the action potential does not enter the soma, but simply continues uninterrupted along the path to the spinal cord. Competing Definitions: 1. Unipolar neurons have but one process from the cell body. However, that single, very short, process splits into longer processes (a dendrite plus an axon). Unipolar neurons are sensory neurons - conducting impulses into the central nervous system. -

Normal Cells of the Cns
NORMAL CELLS OF THE CNS Color index: Slides.. Important ..Notes ..Extra.. Objectives: At the end of this lecture, you should describe the microscopic structure and the function of: 1- Neurons: Cell body (perikaryon). Processes: An axon and dendrites. 2- Neuroglia: Astrocytes. Oligodendrocytes. Microglia. Ependymal cells. Axon: only one Processes Neuron components Dendrites: one or more Cell body (Perikaryon) Types of neurons based on number of processes: Unipolar neuron Has one process only, that divides into two branches; (Pseudounipolar) one acts as a dendrite and the other as an axon. (rounded neuron) e.g. Mesencephalic nucleus of trigeminal nerve Not directly connected to the cell body and dorsal root (spinal) ganglion. Bipolar Neuron Has two processes (one arising from each pole of the cell body) (spindle-shaped neuron) One of them is the dendrite and the other is the axon. like having 2 necks e.g. retina & olfactory epithelium. Multipolar neuron: Stellate Neurons (star shape) Pyramidal Neurons (wide base) Pyriform Neurons Has one axon and multiple - The commonest type. - Distributed in motor area 4 - Pear-shaped dendrites. - Distributed in most areas of CNS of the cerebral cortex. e.g. Purkinje cells of cerebellar -Its outline is irregular in shape e.g. anterior horn cells of the -Neuroglial cells are much more cortex. number than neurons in the CNS spinal cord. they can divide and regenerate normally. Cell body (perikaryon) Cytoplasm: Cytoplasm with mitochondria and ribosomes and rough Nucleus: ER only in dendrites not in axons Single, usually central, rounded and Its main components include: vesicular with prominent nucleolus. Nissl Neuro- Micro- Golgi Mito- Centriole Pigments Other bodies filaments tubles apparatus chondria Depend on age Are * Are Most basophilic intermediate *lipofuscin patches of filaments adult pigment: in rough which are Are neurons old age bundled Endoplasmic found in have Some fat Reticulum together to the cell Surrounds *Melanin (rER) and form the Are only one and rudimentary pigments: in free neurofibrils. -

Guillain-Barre Syndrome (GBS)
Nervous tissue Anatomically Central nervous system (CNS) brain and spinal cord Peripheral nervous system (PNS) - cranial, spinal, and peripheral nerves - ganglia: nerve cell bodies outside the CNS Major cell types Neuron: nerve cell Supporting / Glial cells - Schwann cells, satellite cells (in PNS) - glia/neuroglia (in CNS) Neurone / Neuron Cell body Nucleus Cytoplasm (perikaryon) Process Axon Dendrites Axons (nerve fibers) Axon hillock Terminal boutons Dorsal root ganglia (DRG) nucleus ganglion/ganglia DRG neurons Basic neuron types Multipolar neuron Multiple dendrites Single axons Types: Interneurons Motor neurons Sympathetic neurons Bipolar neuron Single dendrite Single axon Types: Receptor neurons Vision Smell Balance Pseudo-unipolar neuron peripheral Single axon (Stem process) with stem 2 branches: Central process to spinal cord Peripheral process to terminal tissues (muscle, joints, skin et al) functionally: dendrite structurally: axon Type: Dorsal root ganglia (DRG neuron) central Pseudo-unipolar neuron Neuron: ultrastructure Rough endoplamic reticulum (rER) Nissl substance Cytoskeleton Microtubule Intermediate filaments: Neurofilaments Microfilaments: Actin Specialization of neuron/axon Cytoskeleton Axonal transport Neuron: ultrastructure rER: rough ER M: mitochondria L: lysosome G: Golgi Microscopic methods H & E (Hematoxylin and eosin) Nissl method Heavy metal impregnation Golgi, Cajal Thick sections / Spread preparations gold, silver: deposited in microtubules / neurofilaments Immunohistochemistry Microscopic methods: H & E -

Multilineage Differentiation Potential of CNS Cell Progenitors in a Recent
ssing oce & pr B o io i t e B f c Santacroce et al., J Bioproces Biotech 2014, 4:7 h o n l i a q n u DOI: 10.4172/2155-9821.1000186 r e u s o J Journal of Bioprocessing & Biotechniques ISSN: 2155-9821 Research Article Open Access Multilineage Differentiation Potential of CNS Cell Progenitors in a Recent Developed Gilthead Seabream (Sparus aurata L.) Nervous Model Maria Pia Santacroce1*, Antonella Tinelli2, Anna Selene Pastore1, Michele Colamonaco3 and Giuseppe Crescenzo3 1Unit of Aquaculture and Zooculture, Department of Veterinary Medicine, University of Bari “Aldo Moro”, Italy 2Unit of Pathology, Deptartment of Veterinary Medicine, University of Bari “Aldo Moro”, Italy 3Unit of Pharmacology and Toxicology, Department of Veterinary Medicine, University of Bari “Aldo Moro”, Italy Abstract Neural Progenitor Cells (NPCs) have gathered more and more attention in the field of Neural Stem Cells (NSCs). However, the multilineage differentiating behavior of these cells and their contribution to tissue regeneration, almost in lower vertebrate taxa, remain unknown. Since the early 1970s, many comparative studies have been performed using immunocytochemical screening on the brains of several vertebrate taxa, including teleosts, in order to identify these cells, even if the data are sometimes contrasting. This study aims: (1) to investigate in vitro the potential proliferative role of NPCs and Radial Glia Progenitors (RGP) in seabream neurogenesis; (2) to reveal the strict ability of fish NSCs to undertake the multilineage development and differentiation in neurons, astrocytes and oligodendrocytes. By the use of double Immunofluorescence (IF) analysis and phase contrast microscopy, we identified the multilineage differentiation and the exact cell morphology. -

Nerve Cell Impulses
• Localization of Certain Neurons Neurotransmitters Nerve Conduction by: Mary V. Andrianopoulos, Ph.D Clarification: Types of Neuron • There may be none, one, or many dendrites composing part of a neuron. • No dendrite = a unipolar neuron • One dendrite = bipolar neuron • More than one dendrite = multipolar neuron. Multipolar neuron Bipolar neuron Unipolar neuron Localization of Neuron types • Unipolar: – found in most of body's sensory neurons – dendrites are the exposed branches connected to receptors – axon carries the action potential in to the CNS – Examples: posterior root ganglia + cranial nerves – Usually: have peripheral + central connections Localization of Neuron types • Bipolar: – retina, sensory cochlear, vestibular ganglion • Multipolar: (fibers) brain + spinal cord – found as motor neurons and interneurons – neuronal tractsÆ CNS – peripheral nervesÆ PNS Size of Neurons + their localization • Golgi I: – Fiber tracts: brain + spinal cord (PNS + motor) – (i.e., Pyramidal tract + Purkinje cells) • Golgi II: – Cerebral + cerebellar cortex – Often inhibitory – Out number Golgi I – Star-shaped appearance 2° short dendrites Histology of the Nervous System A review of Cell types 1) Neurons - the functional cells of the nervous system 2) Neuroglia (glial cells) - Long described as supporting cells of the nervous system, there is also a functional interdependence of neuroglial cells and neurons a) astrocytes - anchor neurons to blood vessels, regulate the micro-environment of neurons, and regulate transport of nutrients and wastes to and from neurons b) microglia- are phagocytic to defend against pathogens and monitor the condition of neurons c) ependymal - line the fluid-filled cavities of the brain and spinal column and play a role in production, transport, and circulation of the CSF. -

The Nervous System 1) Integration of Body Processes 2) Control of Voluntary Effectors (Skeletal Muscles), and Mediation of Voluntary Reflexes
1 © Jim Swan These slides are from class presentations, reformatted for static viewing. The content contained in these pages is also in the Class Notes pages in a narrative format. Best screen resolution for viewing is 1024 x 768. To change resolution click on start, then control panel, then display, then settings. If you are viewing this in Adobe Reader version 7 and are connected to the internet you will also be able to access the “enriched” links to notes and comments, as well as web pages including animations and videos. You will also be able to make your own notes and comments on the pages. Download the free reader from [Adobe.com] 1 Functions of the Nervous System 1) Integration of body processes 2) Control of voluntary effectors (skeletal muscles), and mediation of voluntary reflexes. 3) Control of involuntary effectors ( smooth muscle, cardiac muscle, glands) and mediation of autonomic reflexes (heart rate, blood pressure, glandular secretion, etc.) 4) Response to stimuli 5) Responsible for conscious thought and perception, emotions, personality, the mind. 2 These functions relate to control of the skeletal muscles discussed in Unit 2 as well as future discussion of reflexes, the brain, and the autonomic nervous system. 2 Structural Divisions of the Nervous System Central Nervous System (CNS) Brain Spinal Cord Peripheral Nervous System (PNS) nerves, ganglia, receptors 3 The central nervous system develops from the neural tube, while the peripheral nervous system develops from the neural crest cells. 3 Functional Divisions of the Nervous System 1) The Voluntary Nervous System - (a.k.a. somatic division) willful control of effectors (skeletal muscles), and conscious perception. -

Human Anatomy and Physiology I Laboratory
Human Anatomy and Physiology I Laboratory Histology of Nervous Tissue and The Spinal Cord This lab involves two laboratory exercises: 1) “Histology of Nervous Tissue”, and 2) “Spinal Cord, Spinal Nerves, and the Autonomic Nervous System”. Complete the Review Sheet for the entire first exercise, and the portion pertaining to the spinal cord for the second. The remainder of the second exercise will be completed in the next lab on the spinal and peripheral nerves. The quizzes are separate as well: for this lab take the quiz on nervous tissue and the spinal cord. Alternately, your instructor may have you turn in drawings of nerve tissue in lieu of the Review Sheets. Use the Virtual Microscope or other histology sites for images of nerve tissue. Click on the sound icon for the audio file (mp3 format) for each slide. There is also a link to a dowloadable mp4 video which can be played on an iPod. 1 Cells of the Nervous System: Glial Cells (neuroglia) and other supportive cells: These provide supportive functions for the nervous system. And new information stresses the functional interdependence of glial cells and neurons. Neurons: these are the functional cells of the nervous system, i.e. they conduct the impulses. 2 CNS Neuroglia a) Astrocytes - these cells anchor neurons to blood vessels, regulate the micro-environment of neurons, and regulate transport of nutrients and wastes to and from neurons. They are part of the blood-brain barrier. b) Microglia - these cells are phagocytic to defend against pathogens. They may also monitor the condition of neurons. -

The Nervous System
THE NERVOUS SYSTEM The nervous system primarily consists neurons and neuroglia. The functional unit of the nervous system are the neurons. A neuron can be defined as a nerve cell. The unique structure of neurons makes them specialized for receiving and transmitting electrical impulses throughout the body. The neuron acts like a miniature self-contained information processor. It receives inputs, processes information, and generates outputs. Neuron as the functional unit of nervous system was demonstrated by R.Y. Cajal while the nerve cell or the neuron was first described by Camillo Golgi in 1873. 3.1Structure of neuron The membrane, which surrounds the nerve cell, is made up of a double layer of lipid and contains protein molecules that play many important roles in transporting and blocking substances from coming in and out of the cell. A neuron has three main functional parts: the structure most associated with receiving signal is the dendrite, the body or soma , which accumulates signals coming from the input (dendrites) and which produces at the axonal hillock a series of bursts (impulses) when the accumulated signal reaches a critical threshold. These impulses are propagated to other neurons through an output called axon. The structure of neuron is described below: a) Neurocyton The cell body (soma or neurocyton or perikaryon) contains a granular cytoplasm due to the presence of basophilic granules called Nissl’s bodies. Nucleus is single and centrally located. Other cell organelles present are mitochondria, Golgi apparatus, ribosomes, endoplasmic reticulum , neurofibrillae and centrioles. The Nissl’s granules which are sometimes referred to as chromatophilic substances are composed of ribonucleoproteins are produced in the nucleus and play important role in.