Application of Additives in Chilled and Frozen White Fish Fillets - Effects on Chemical and Physicochemical Properties –
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

REPORT on the NORWEGIAN FROZEN FISH FILLET Industryl/ by R
August 1953 Washington 25, D.C. Vol. I 5 , No.8 : REPORT ON THE NORWEGIAN FROZEN FISH FILLET INDUSTRyl/ By R. T. Wh i teleather* FILLETING INDUSTRY IN GENERAL The fisheries that support the groundfish fillet industry in Norway are lo cated in the northern part of the country principally from the Lofot Islands area northward to Hammerfest in Finnmark. These fisheries, particularly those for cod, are generally ranked among the most prolific in the world. In a normal year the Lofot fisheries alone from January through March yield from 80,000 to 120,000 met ric tons of large cod. Notwi t hst anding this, the 1953 spring fishery for cod was disastrous owing to violent storms and almost continuous bad weather for the two- onth period which comprised most of the season. No trawlers operate in t he Lofot cod fishery. The purse seiners, gill netters, ong liner., and hand liners used are dependent to a great extent on reasonably air weather conditions. As a result of the curtailed fishing because of the poor weather during the 1953 ( orwegian Landings of p-r1nc-ipal Species Used in Fillet season, the landings of Industry. 1948- 52 headed and gutted cod asof Species 19521/ 11951 19501194911948 March 30 amounted to only '. Thousands of r.~etric Tons) about 25,000 tons, one of octY (headed and gutted ) . 218 1 249 1961176\ 179 the poorest showings during addock •••••.••• do . ..... 22 18 18 21 25 the past 50 years. Since cean catfish ••• do. ..... 5 6 5 3 1 }'larch is usually considered )cean perch (round) •. -

Total Mercury and Copper Concentrations in Lake Trout and Whitefish Fillets from Lake Superior
Total Mercury and Copper Concentrations in Lake Trout and Whitefish Fillets From Lake Superior Activity: 19 -23 By Kory Groetsch Environmental Section Biological Services Division INTRODUCTION Mercury and copper are the two most toxic heavy metals to fish. Furthermore, methyl mercury can accumulate in fish muscle tissue to levels that, consumed on a regular basis, may pose a risk to human health. Both of these metals are released during copper mining and ore processing. Copper mining and processing were a major industry in the Keweenaw Peninsula of Michigan during the mid-1800's. Mining processes which involved heating the ore, released mercury as a bi-product into the air. Furthermore, the unused portion of the ore after the desired minerals were removed (i.e. tailings) were dumped off the shoals of the Keweenaw Peninsula into Lake Superior. These tailing have formed large sholes referred to as stamp sands. Because the extraction of copper from the ore is not a 100% efficient process, it is reasonable to assume that copper, as well as mercury, were in the tailings released into the waters around the Keweenaw Peninsula. The waters around the Keweenaw Peninsula are the location of several lake trout and whitefish spawning reefs. The lake trout and whitefish populations around the Keweenaw Peninsula are sustained by the fish reproduction that occurs on these reefs. These lake trout and whitefish populations have significant cultural and economic importance to the Anishinabe. The purpose of this study was to determine the total mercury and total copper concentrations in the muscle tissue (i.e. fillet) of lake trout and whitefish, in size ranges commonly caught by tribal fishermen around the Keweenaw Penisula. -

Silverfin (Asian Carp) Recipes
Chef Philippe Parola and Silverfin Promotion, LLC The Asian Carp Invasion Solution A Letter from Chef Philippe After almost two years of research and development focused on the Asian carp invasion solution, we proudly have identified and engineered the necessary food technology to process Asian carp into boneless, pre-cooked, microwaveable, healthy, U.S. wild-caught, affordable, value-added Silverfin fillets. Our packaging concepts are consumer friendly and easy to identify as a premiere U.S. fish product under the Silverfin Craze brand name. To be able to successfully remove Asian carp, we must create a strong incentive to put present and future fishermen to work. This incentive will be easily achieved by marketing our value-added fish products in national and international food market places. Furthermore, our formula will sustain itself and generate multiple tax revenues to Local, State and Federal governments. Our clear and simple solution includes processing up to 80,000 pounds of raw fish per day. This will boost fresh water fisheries and local economies by creating permanent and part time jobs. It will also help control the Asian carp population, and prevent a total take-over by these invasive species which would displace our native fish. Our processing concept will be able to handle other invasive fish species such as Black carp and Snakehead . Removing so many of these large jumping fish from American waters on a daily basis will also reduce the danger of someone being seriously injured, or even killed, by these fish when they leap from the water. When we met with Mr. -

Influence of Controlled Freezing-Point Temperature on the Freshness and Taste of Pickled and Dried Grass Carp
Advances in Engineering Research (AER), volume 130 5th International Conference on Frontiers of Manufacturing Science and Measuring Technology (FMSMT 2017) Influence of controlled freezing-point temperature on the freshness and taste of pickled and dried grass carp LiYan Cai a, JinQing Wanb 1College of Food Science and Technology, Shanghai Ocean University, Shanghai 201306, China; [email protected],*Corresponding author Email: [email protected] Keywords: fillets, drying, freshness, flavor, controlled freezing-point temperature Abstract. Pickling and drying can help aquatic products with their flavor which is highly related to temperature. Taking grass carp fillets as the research objects, quality changes were studied during pickling, drying and storage under controlled freezing-point temperature (CFT:-0.5±0.5℃) and 12 ±0.5℃. Results showed that total volatile basic nitrogen (TVB-N) value of grass carp fillets under CFT were significantly lower than that in 12℃. TVB-N value of fillets was only 29.84 mg N/100g under CFT after 75 days storage and reached 30.88mg N/100g under 12℃ only after 35 days storage. Inosine 5-monophosphate (IMP) of the fillets both increased firstly and then decreased smoothly and they all increased to the highest level on first day with the value of 169.30 mg/100g under CFT and 156.20 mg/100g under 12℃ respectively. During the storage, IMP of the fillets decreased much slower and maintained a higher level. Compared to fillets under 12℃, free amino acids and equivalent umami concentration (EUC) of fillets under CFT had significant increase (P<0.05). At the end of the storage, EUC of fillets was 1.568 MSG/100g under CFT and 0.898 MSG/100g under 12℃. -
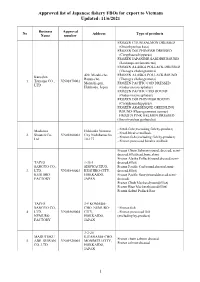
Approved List of Japanese Fishery Fbos for Export to Vietnam Updated: 11/6/2021
Approved list of Japanese fishery FBOs for export to Vietnam Updated: 11/6/2021 Business Approval No Address Type of products Name number FROZEN CHUM SALMON DRESSED (Oncorhynchus keta) FROZEN DOLPHINFISH DRESSED (Coryphaena hippurus) FROZEN JAPANESE SARDINE ROUND (Sardinops melanostictus) FROZEN ALASKA POLLACK DRESSED (Theragra chalcogramma) 420, Misaki-cho, FROZEN ALASKA POLLACK ROUND Kaneshin Rausu-cho, (Theragra chalcogramma) 1. Tsuyama CO., VN01870001 Menashi-gun, FROZEN PACIFIC COD DRESSED LTD Hokkaido, Japan (Gadus macrocephalus) FROZEN PACIFIC COD ROUND (Gadus macrocephalus) FROZEN DOLPHIN FISH ROUND (Coryphaena hippurus) FROZEN ARABESQUE GREENLING ROUND (Pleurogrammus azonus) FROZEN PINK SALMON DRESSED (Oncorhynchus gorbuscha) - Fresh fish (excluding fish by-product) Maekawa Hokkaido Nemuro - Fresh bivalve mollusk. 2. Shouten Co., VN01860002 City Nishihamacho - Frozen fish (excluding fish by-product) Ltd 10-177 - Frozen processed bivalve mollusk Frozen Chum Salmon (round, dressed, semi- dressed,fillet,head,bone,skin) Frozen Alaska Pollack(round,dressed,semi- TAIYO 1-35-1 dressed,fillet) SANGYO CO., SHOWACHUO, Frozen Pacific Cod(round,dressed,semi- 3. LTD. VN01840003 KUSHIRO-CITY, dressed,fillet) KUSHIRO HOKKAIDO, Frozen Pacific Saury(round,dressed,semi- FACTORY JAPAN dressed) Frozen Chub Mackerel(round,fillet) Frozen Blue Mackerel(round,fillet) Frozen Salted Pollack Roe TAIYO 3-9 KOMABA- SANGYO CO., CHO, NEMURO- - Frozen fish 4. LTD. VN01860004 CITY, - Frozen processed fish NEMURO HOKKAIDO, (excluding by-product) FACTORY JAPAN -

EPA Releases Report on Fish Contamination in U.S. Lakes and Reservoirs
United States Office of Water EPA-823-F-09-008 Environmental Protection Agency 4305T September 2009 EPA Releases Report on Fish Contamination in U.S. Lakes and Reservoirs EPA is releasing the final report for the National Study of Chemical Residues in Lake Fish Tissue, a statistically-based national survey of contaminants in fish from lakes and reservoirs in the lower 48 states. For four years, EPA worked with 47 states, three tribes, and two other federal agencies to collect fish from 500 lakes and reservoirs selected randomly from the estimated 147,000 target population of lakes and reservoirs in the 48 states. Analysis of fish samples included 268 persistent, bioaccumulative, and toxic (PBT) chemicals, most notably mercury, polychlorinated biphenyls (PCBs), and dioxins and furans. Results show that mercury and PCBs were detected in every fish sample from all 500 lakes and reservoirs. Mercury concentrations in fish fillet samples exceeded EPA’s recommended tissue-based water quality criterion of 0.3 ppm at 49% of the sampled population of 76,559 lakes. Background EPA completed the largest national freshwater fish contamination survey conducted to date in the United States, the National Study of Chemical Residues in Lake Fish Tissue (or National Lake Fish Tissue Study). Two features make this study unique. It is the first national freshwater fish contamination survey to have sampling sites selected according to a statistical design, and it includes the largest set of chemicals ever studied in lake fish. The statistical survey design allowed EPA to estimate the percentage of lakes and reservoirs in the lower 48 states with fish tissue concentrations above levels of potential concern for human or ecosystem health. -

A Complete Listing of Our Frozen Seafood Items
IWC FOOD SERVICE 9/1/2021 Product Listing Frozen Seafood Item No Pack Size Brand Category Description 14030 1 10.35LB VIKING FISH FISH POLLOCK POT CRUNCH BRD 3.6 WG 2852 4 2.5 LB SEA BEST CALAMARI SQUID TENTACLE TUBE 3-5" 2971 6 2 LB TAMPA MAID CALAMARI CALAMARI RING BRD 40-50 CT ITALIAN SEAS 4029 24 4 OZ SEA WATCH CLAMS CLAM STRIP BRD 4049 1 6 LB SEA WATCH CLAMS CLAM STRIP BRD 14006 10 1 LB HARBOR SEAFOOD CLAMS SPEC CLAMS BROWN N SHELL 17-22 COOKED 4096 1 30 LB CRUST/MARITIME CRAB CRAB SNOW LEG 5-8OZ CANADIAN 4116 4 2.5 LB SEA SHELLS CRAB CRABMEAT FLAKED BLENDED 4304 6 12/3 OZ CAP'N JOE CRAB CRAB DEVILED STUFFED N SHELL(6-12CT) 4305 53 3 OZ MRS FRIDAY CRAB CRAB CAKE BRD 4307 1 9 LB ICELANDIC CRAB CRAB CAKE MARYLAND STYLE 48 CT 4317 4 2.5 LB KING & PRINCE CRAB CRAB CAKE GOURMET 14041 12 1 LB BOSS CRAB CRAB JUMBO LUMP PASTERUIZED BLUE REF 14042 12 1 LB SEA BEST CRAB CRAB LUMP MEAT PASTERUIZED RED FRIG 14043 12 1 LB CAP CHARL/SHERI CRAB CRAB CLAW MEAT PASTERUIZED BLUE REFRIG 14413 6 1 LB PHILLIPS CRAB CRAB CLAW MEAT PASTERUIZED BLUE REFRIG 29127 12 2.5 LB SEA BEST CRAB CRAB IMT FLAKE 4182 24 1 LB CRAW ROYAL/BORD CRAWFISH CRAWFISH TAIL MEAT CHINESE 80-100 4012 1 10 LB EARLY DAWN/TALA FISH FISH TILAPIA FILLET 3-5 OZ IQF 4031 1 10.35LB VIKING FISH FISH POLLOCK BRD P/C 3.6 OZ WG WEDGE 2M 4044 1 10 LB REST PRIDE FISH CATFISH NUGGET BRD 1-2 OZ 4054 160 1 OZ VIKING FISH FISH POLLOCK NUGGET WG 1OZ 4=2MT 4064 1 10 LB TRIDENT FISH FISH POLLOCK STICK BAJA WG 1OZ 4 2MT 4071 53 3 OZ SAMBAND FISH FISH POLLOCK P/C BRD SQUARE 418317CN 4081 1 10 LB FISHERY -

Developments
16 COMMERCIAL FISHERIES REVIEW Vol. 22 , No. 3 AND DEVELOPMENTS American Samoa firms which accounted for 91 percent of the 1958 production) amounted to 27 5, 000 American Samoa Tuna Landings, 1958-59 short tons--a gain of 48,000 tons a s com , December Jan.-TIec. pared with 1958. The marine - animal oil Species 1959 1858 1959 1958 yield amounted to 24.4 million gallons-- . (1,000 Lbs.) . 2.4 million gallons above 1 958 . Albacore 2, 655 2,452 22,474 22 , 251 Yellowfin 238 613 4,017 5,537 Imports of fish meal and scrap during Big-eyed 63 53 982 1,060 1959 totaled 133,000 tons --33, 000 tons Skipjack 1 - 15 - more than in 1958. A sharp increas e of Total 2,957 3,118 27,488 28,848 82.8 percent in the imports of fish s olu Note: Most of these tuna were landed by Japanese vessels; bles also occurred from 1958 to 1959. a small amount bv South Korean vessels. Exports of fish and fish - li ver oils t otal ed 144.5 million pounds during 1959 --a gain of 50.5 million pounds as compared with 1958. Note: See Commercial Fisheries Review, Apri1 1959, p. 37. Byproducts U. S. FISH MEAL, OIL, AND SOLUBLES PRODUCTION, 1959: During 1959, fish meal and scrap production (based on information from California Ill. S. Production of Fish Meal, Oil, and Solubles, 1958-59 AERIAL CENSUS OF COMMERC IA L Product 1959 1958 ABALONE FISHING CONTINUED: PreliminarY Revised Airplane Spotting F light 59 - 23-Abalone: The Unit . -

Vietnam's Tra and Basa Fish Exports to the Us
FULBRIGHT ECONOMICS TEACHING PROGRAM CE03-52-8.0 ____________________________________________________________________________ Feb 27, 2003 NGUYEN XUAN THANH CATFISH FIGHT: VIETNAM’S TRA AND BASA FISH EXPORTS TO THE US On August 8 th , 2002 a meeting of the US International Trade Commission (ITC) concluded with the following statement: "Based on the preliminary findings, we determine that there is a reasonable indication that the domestic industry producing frozen catfish fillets, whether or not breaded or marinated, is threatened with material injury by reason of subject imports from Vietnam that are allegedly sold in the United States at less than fair value.” This decision came forty days after the Catfish Farmers of America (CFA), a trade association of US catfish farmers, accused 53 Vietnamese fishery exporters and processors of dumping frozen tra and basa fillets on the US market, causing major damage to local catfish producers. Thus, the initial round of the first dumping case between the US and Vietnam ended. The case was then passed to the US Department of Commerce for a dumping investigation. The verdict is expected in July 2003 if the case continues till its last stage. If the verdict is in favor of the CFA, i.e. the petitioner, then imports of frozen tra and basa fillets from Vietnam will be subject to antidumping duties as high as 191 percent. This is in contrast to the current rate of zero percent. Since the dumping charge was initiated shortly after the US-VN BTA became effective, it drew enormous attention from the government and the business community in Vietnam. There is particular concern for the impact this case would have on the livelihood of almost 200,000 farmers in the Mekong Delta. -

Improved Hot Smoke Processing of Chub Mackerel (Scomber Japonicus) Promotes Sensorial, Physicochemical and Microbiological Characteristics
applied sciences Article Improved Hot Smoke Processing of Chub Mackerel (Scomber japonicus) Promotes Sensorial, Physicochemical and Microbiological Characteristics Md. Masud Rana 1,†, Md. Mohibbullah 1,† , Na Eun Won 2, Md. Abdul Baten 1 , Jae Hak Sohn 2, Jin-Soo Kim 3,* and Jae-Suk Choi 2,4,* 1 Department of Fishing and Post Harvest Technology, Sher-e-Bangla Agricultural University, Dhaka 1207, Bangladesh; [email protected] (M.M.R.); [email protected] (M.M.); [email protected] (M.A.B.) 2 Seafood Research Center, Silla University, #605, Advanced Seafood Processing Complex, Wonyang-ro, Amnam-dong, Seo-gu, Busan 49277, Korea; [email protected] (N.E.W.); [email protected] (J.H.S.) 3 Department of Seafood and Aquaculture Science, Gyeongsang National University, Tongyeong-si 53064, Korea 4 Department of Food Biotechnology, Division of Bioindustry, College of Medical and Life Sciences, Silla University, Busan 46958, Korea * Correspondence: [email protected] (J.-S.K.); [email protected] (J.-S.C.); Tel.: +82-55-772-9146 (J.-S.K.); +82-51-248-7789 (J.-S.C.) † M.M.R. and M.M. contributed equally. Abstract: Chub mackerel (CM), Scomber japonicus, is a commercially important fish species in pacific Citation: Rana, M.M.; Mohibbullah, countries including South Korea and its rapid quality deterioration by various spoilage mechanisms M.; Won, N.E.; Baten, M.A.; Sohn, while marketed has been reported, leading to a dramatic decline of the market price. To overcome J.H.; Kim, J.-S.; Choi, J.-S. Improved this problem, a combination of superheated steam roasting (270 ◦C for 4 min) and hot smoking Hot Smoke Processing of Chub (70 ◦C) on CM fillets was applied to impart extending shelf-life at the market level. -

Fishery Sector Overview 2017
FISHERY SECTOR OVERVIEW 2017 RUSSIAN AND NORTH-WEST REGION FLANDERS INVESTMENT & TRADE MARKET SURVEY Russian and North-West Region Fishery Sector Overview 2017 June 2017 Frank Van Eynde, Vlaams economisch vertegenwoordiger Flanders Investment & Trade Saperny Pereulok 11 191014 Saint Petersburg | Russia [email protected] Table of contents Sector Profile .......................................................................................................................................... 2 Fleet and Infrastructure ......................................................................................................................... 3 Fish Export and Import .......................................................................................................................... 4 Fish Production: Wild Catch .................................................................................................................. 5 Fish Production: Aquaculture ................................................................................................................ 7 Fish Processing ....................................................................................................................................... 9 Fish Consumption ................................................................................................................................. 11 Main Problems in the Russian Fishery Sector .................................................................................... 11 Russian Fishery Development Strategy -

THE OCCURRENCE of Pseudomonas Sp in GROUPER FISH FILLETS
c- Bmed Approach for Food Safely Mdnageme~it Proceeding qf internut tonal Sem I nor Current Issues and Challenges in Food Sqfeiy . P., Woutersen, R. A., Feron, V. J., Hollanders, V. H., Falke, H. E., & OF SP IN CIary, J. 1. 1989. TWO-Year ~rinking-water study of THE OCCURRENCE Pseudomonas Formaldehyde in Rats. Food Chem. Toxicol., 27(2):77-87. GROUPER FISH FILLETS (Plectroporna , M., Naito, K., & Kurokawa, Y. 1989. Chronic Toxicity Study on leopordus) AT BONE PANTE Formaldehyde Administered Orally to Rats. Toxicology, 56(1]:79-86. Rieny Sulistijowati* and Royhan Alhasen** * Universitas Muhamrnadiyah Gorontalo **Universitas Negeri Gorontalo [email protected] ABSTRACT Study on the occurrence of Psetrdomonas sp., associated with grouper fish (PIectroporna leopardus) was done. The research aimed to know the occurrence of Pseudomor~assp it1 fresh grouper fish (P. leopardus) fillets chilled in ice. The fish samples were taken from grouper fish farm located at Bone Pante village (separated into two sampling area 1 and 11). The result showed that both of total plate count [TPC) and total Pseurlomonas (TP) of grouper fish fillet were lower in samples taken from sampling area I than those taken from sampling area I I. The TPC of sample from sampl~ngarea 1 ranged between 2,1 x 1 O4 CFU/g to 2,8 x 1 04,while from sarl~plingarea II it ranged between 1,9 x 10TCC;Ug to 5,Z x 104 CFll/g. Total P~~rrdornonossp. of sample from sampling area I ranged between 1.8 x103 CFIJ/g to 4,4 x103 CFU/g, while from sampling area II, it ranged between 8,O xlO2 CFU/g to 3,7 x lo3 CFU/g.